The AACR is always bustling every year, and this year I am particularly focused on the progress of dual payload ADCs. I have written several articles on this topic before, and just a few days ago, I was discussing with my supervisor that “I haven’t seen any dual payload ADCs advancing to clinical trials yet,” but yesterday I saw that Kanghong Pharmaceutical’s KH815 has already entered Phase I (I originally thought it would be Araris). I summarized and found that this year at AACR, in addition to dual payload ADCs, there are also several dual antibody dual payload ADCs making their debut, intensifying the competition.1、Araris Biotech AG
7334 / 20 – Targeting Nectin-4 with a first-in-class triple MMAE/dual TOP1i payload ADC showing synergistic and durable activity across all target expression levels and favorable tolerability
LB138 / 17 – A novel dual-TOP1i ADC targeting NaPi2b designed for high efficacy and tolerability
First, regardingAraris, since the announcement of their collaboration with domestic and foreign pharmaceutical companies and Dapeng Pharmaceutical, this company has attracted widespread attention. Their core technology isAraLinQ™, which utilizes microbial transglutaminase (MTG) to chemically modify the Q295 on the antibody Fc. However, this method generally requires deglycosylated IgG to be effective, so it either requires PNGase F to deglycosylate N297 or mutating N297 to alanine (N297A) to eliminate glycosylation. But deglycosylated IgG loses ADCC effects, and due to increased protein degradation and aggregation tendencies, it may reduce the stability of the antibody.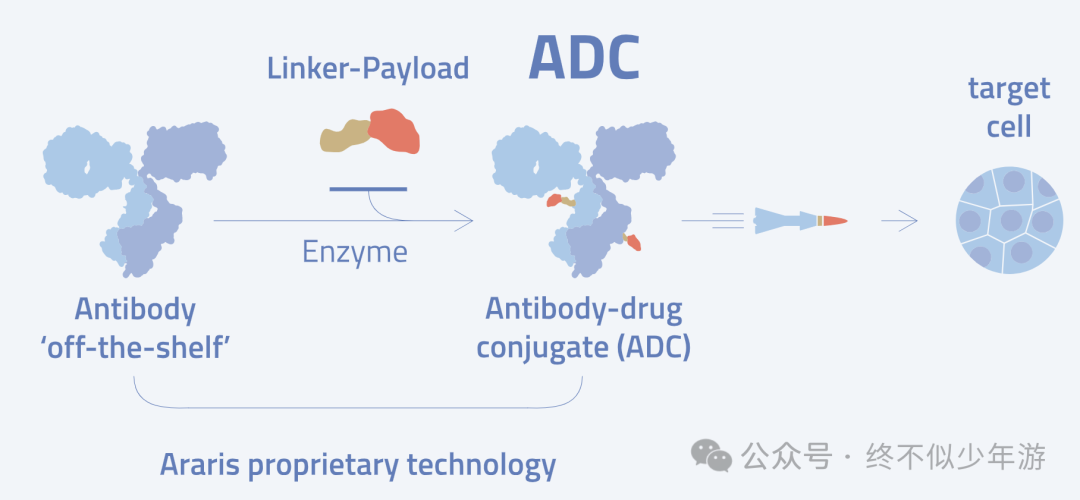 Araris has published in《ChemBioChem》 a method for directly modifying the Q295 of natural IgG using positively charged short peptides like RAK. By using RAK containing azide and thiol groups, ADCs with different DAR values can be obtained.
Araris has published in《ChemBioChem》 a method for directly modifying the Q295 of natural IgG using positively charged short peptides like RAK. By using RAK containing azide and thiol groups, ADCs with different DAR values can be obtained.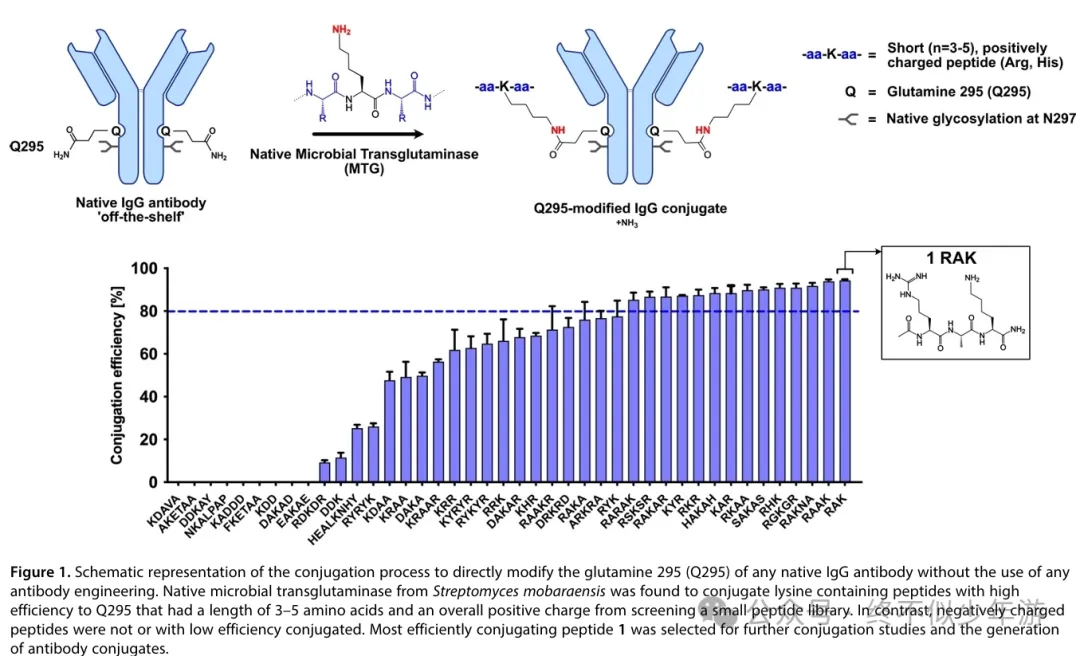 (doi.org/10.1002/cbic.202400511) Araris announced two new ADCs at AACR this year, one is a triple payload ADC targeting Nectin-4, which uses a combination of two different Topo1i and MMAE, with DAR values ofTopo1i (2)+ Topo1i (2)+MMAE(2). The NaPi2b ADC currently does not have an abstract available on its official website, so let’s first look atNectin-4. The abstract does not detail the specific information of the two Topo1i, but based onAraris’s description of another dual-TOP1i DAR4 HER-2 ADC at the 2024 AACR, they are composed of a non-bystander effect DAR2 exatecan (which increases the toxin content in tumor cells and enhances killing activity) and a bystander effect DAR2 exatecan (which addresses tumor heterogeneity and low antigen expression). As forNectin-4’s triple payload ADC, whether these twoTopo1i will follow the same technical route needs to be confirmed later.
(doi.org/10.1002/cbic.202400511) Araris announced two new ADCs at AACR this year, one is a triple payload ADC targeting Nectin-4, which uses a combination of two different Topo1i and MMAE, with DAR values ofTopo1i (2)+ Topo1i (2)+MMAE(2). The NaPi2b ADC currently does not have an abstract available on its official website, so let’s first look atNectin-4. The abstract does not detail the specific information of the two Topo1i, but based onAraris’s description of another dual-TOP1i DAR4 HER-2 ADC at the 2024 AACR, they are composed of a non-bystander effect DAR2 exatecan (which increases the toxin content in tumor cells and enhances killing activity) and a bystander effect DAR2 exatecan (which addresses tumor heterogeneity and low antigen expression). As forNectin-4’s triple payload ADC, whether these twoTopo1i will follow the same technical route needs to be confirmed later. For proof-of-concept and comparison to T-DXd, trastuzumab was used as the targeting antibody. The Araris ADC was designed to combine two features in one ADC to maximize tumor-specific activity by using two different exatecan-based payloads: one that is able to exert bystander activity to address tumor heterogeneity and low-target expression and one that accumulates in cancer cells (no bystander activity) to achieve greater potency.Importantly, the non-bystander exatecan showed an increased intracellular concentration (up to 4x) and the bystander capable exatecan demonstrated high bystander activity in co-cultured, target-negative cell lines.2、Chengdu Kanghong Pharmaceutical
For proof-of-concept and comparison to T-DXd, trastuzumab was used as the targeting antibody. The Araris ADC was designed to combine two features in one ADC to maximize tumor-specific activity by using two different exatecan-based payloads: one that is able to exert bystander activity to address tumor heterogeneity and low-target expression and one that accumulates in cancer cells (no bystander activity) to achieve greater potency.Importantly, the non-bystander exatecan showed an increased intracellular concentration (up to 4x) and the bystander capable exatecan demonstrated high bystander activity in co-cultured, target-negative cell lines.2、Chengdu Kanghong Pharmaceutical
1586 / 2 – KH815, a novel dual-payload TROP2-directed antibody-drug conjugate, shows potent antitumor efficacy in pre-clinical tumor model
Kanghong Pharmaceutical presented two dual payload ADCs at this AACR. The first is the aforementionedKH815: using the TROP-2 antibody hRS7 as a scaffold, it combinesTopo1i (DAR 4) and RNA pol 2 inhibitor (DAR 3.5), utilizing cysteine and glycoside antibody conjugation technology, with NHP HNSTD reaching 40 mg/kg/dose.
1587 / 3 – A novel dual-payload HER3-directed antibody-drug conjugate, shows potent antitumor efficacy in HER3-low in vivo tumor models
The other isKHN922, which uses patritumab as the antibody scaffold, also combiningTopo1i (DAR 4) and RNA pol 2 inhibitor (DAR 3.5). In NHP,HNSTD reached 50 mg/kg/dose.3、YilianMediLink
1804 / 23 – An innovative dual-payload ADC combining topoisomerase 1 inhibitor and a tubulin inhibitor efficiently overcomes drug resistance
Yilian continues to useTMALIN® technology as the core, with trastuzumab as the antibody, conjugating Topo1i (DAR 8) and tubulin inhibitor (DAR 2):the tubulin inhibitor is conjugated at specific lysine sites on the antibody, whileTopo1i is conjugated to free cysteine thiols through TMALIN® technology.4、Caronia
5451 / 9 – JSKN021, an innovative site-specific dual-payload bispecific antibody drug conjugate targeting EGFR and HER3 exhibits potent preclinical activities
Caronia’sJSKN021 targets EGFR x HER3, combining glycosylatedTopo1i (DAR 4) and MMAE (DAR 2).5、Hangzhou Duoxi
2872 / 11 – DXC018: A novel HER2 dual-payload bispecific ADC with promising potential for gastrointestinal cancers and other solid tumors
DXC018 is a dual epitope HER antibody, with toxins beingTopo1i and anti-metabolite inhibitors, DAR value unknown.6、Shanghai Qinhuali Biotechnology 1805 / 24 – Dual-payload TME-activated ADC platform Shanghai Qinhuali Biotechnology presented ADCs targetingHER2 IMD526, ADC targeting PD-L1 IMD2126, and ADC targetingEGFR x TROP2 IMD2113, with various attempts in toxins, includingTopo1i + TLR7/8 agonist; among them,IMD2113 is a dual antibody dual payload ADC.7、Sutro
2870 / 9 – Enhancing Topo1i ADC efficacy: development of homogeneous dual-payload ADCs combining Topo1i with microtubule inhibitors or PARP inhibitors
Sutro’s dual payload has been introduced before (《Self-immune Volume Three Antibody, ADC Volume Dual Payload), Topo1i + PARPi is one of the attempts.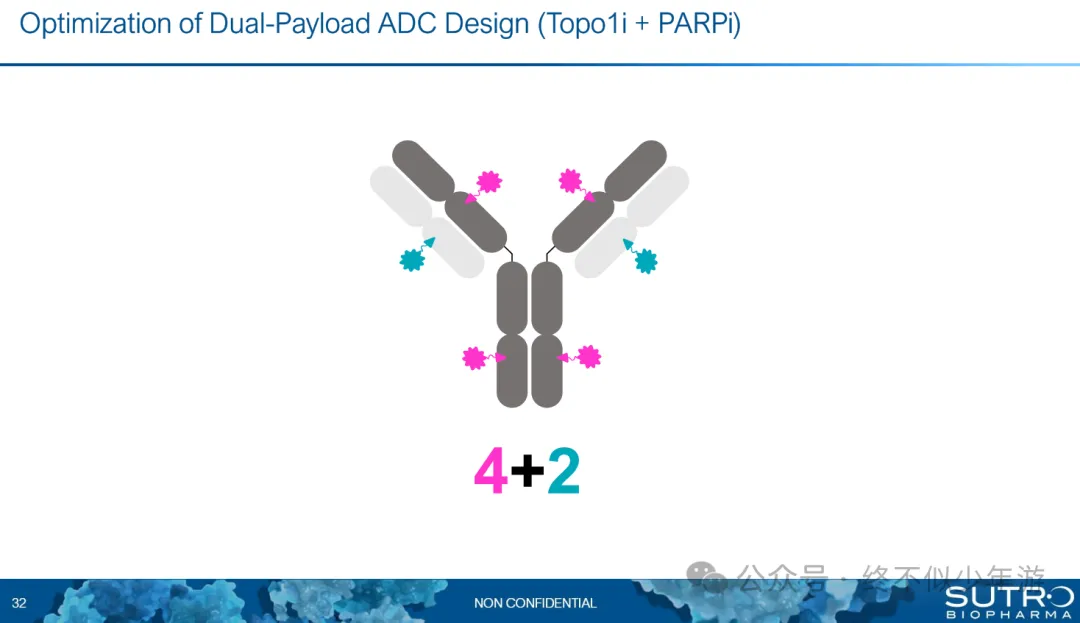 8、Pinotbio
8、Pinotbio
6753 / 19 – Exploring new approaches to widening of the therapeutic index of ADCs with dual-payload design and combination with a novel HMA
Pinotbio showcased aHER2-targeted ADC, with toxins selected as Topo1i and tubulin inhibitor, DAR value unknown.9、Celltrion Pharm
6755 / 21 – Discovery of the synergistic dual-payload antibody-drug conjugate (CTPH-02) by combination of MMAE and novel payloads
South Korea’sCelltrion announced a HER-2 targetedtrastuzumab antibody, with one of the toxins seemingly being MMAE, while the other is uncertain.10、Tuojiyiyuan
LB021 / 21 – TJ102: A promising bispecific dual-payload ADC targeting CDH6 and folate receptor alpha (FRα) for the treatment of ovarian and kidney cancers
The abstract has not been published with detailed information, but the title suggests it is aCDH6/FRα dual antibody dual payload ADC.11、Acepodia
1785 / 4 – Development of dual-payload anti-GPC3 antibody-drug conjugate by dual-payload antibody conjugation (AD2C) platform for hepatocellular carcinoma treatment
Acepodia’s is aGPC3-targeted dualpayload ADC (anti-GPC3 AD2C), with the toxinunknown. On January 8, at the beginning of the year, Baiyao announced a collaboration with Acepodia to advance the development of dual antibody dual payload ADCs (BsAD2C), whose AD2C platform also does not require engineering modifications to the antibody to conjugate two (or more) different toxins with the antibody.
Outside of AACR, Innovent Biologics also announced its dual payload ADC plans. According to this year’s information from the JPM conference on their official website, in addition to the CEACAM5-targeted IBI3020, there may be another dual antibody dual payload ADC, awaiting further information to be released.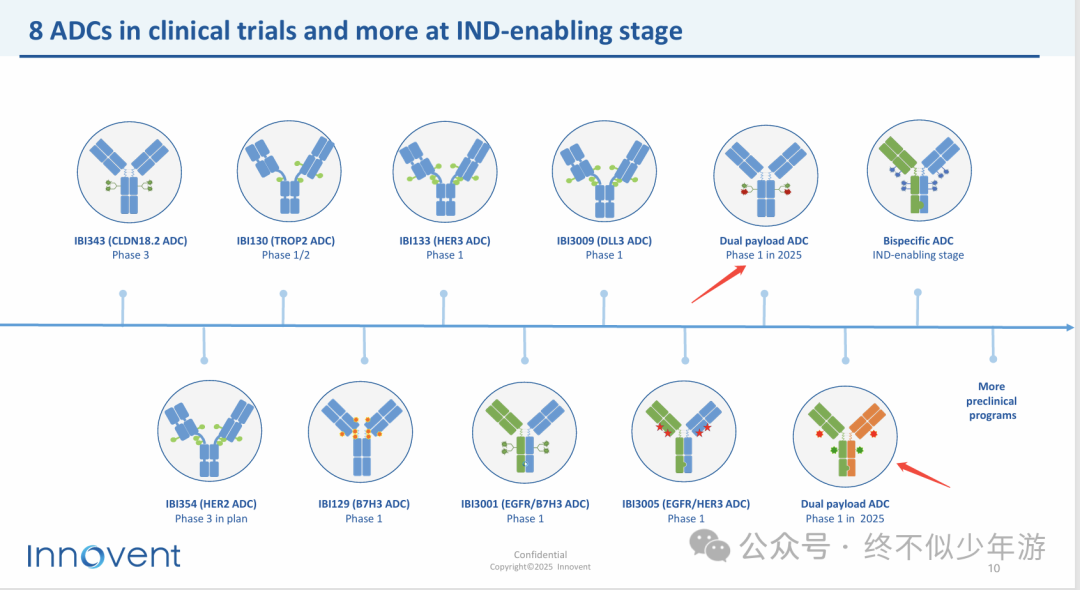
 References:1、https://aacrjournals.org/cancerres/article/84/6_Supplement/2600/740443/Abstract-2600-Novel-dual-TOP1i-ADC-inducing2、Innovent Biologics official website and WeChat public account
References:1、https://aacrjournals.org/cancerres/article/84/6_Supplement/2600/740443/Abstract-2600-Novel-dual-TOP1i-ADC-inducing2、Innovent Biologics official website and WeChat public account