Antibody-drug conjugates (ADC) are a class of targeted cancer therapies that combine the high specificity of monoclonal antibodies with the potent activity of small molecule cytotoxic drugs to enhance tumor targeting and reduce side effects. Compared to traditional antibodies or antibody fragments, ADCs theoretically have higher efficacy due to their ability to release highly active cytotoxins within tumor tissues. The development of ADCs has gone through three stages:
First Generation ADCs: The antibodies used in first-generation ADCs were murine or chimeric antibodies, and the linkers were not stable enough; due to non-specific coupling, the drug-to-antibody ratio (DAR) was uncontrollable. These characteristics resulted in poor efficacy, stronger toxicity/side effects, high immunogenicity, and shorter half-lives.
Second Generation ADCs: Second-generation ADCs improved upon the first generation by selecting antibodies with better affinity for the antigen, reducing cross-reactivity with normal tissues, and humanizing to decrease immunogenicity. The small molecule toxins chosen were more effective, such as MMAE and MMAF. The linkers were either degradable or non-degradable. Compared to the previous generation, they exhibited higher targeting, better efficacy, and lower immunogenicity. However, they still had significant side effects, developed resistance, and high DAR values that led to rapid clearance of the drug.
Third Generation ADCs: Third-generation ADCs continued to improve upon the second generation by utilizing small molecule toxins to achieve site-specific coupling with human monoclonal antibodies, resulting in more uniform DAR values and a variety of differentiated small molecule toxins to choose from. Compared to the first two generations of ADCs, the stability and pharmacokinetics have greatly improved, leading to higher drug activity and lower toxicity.
With the development of third-generation ADCs, antibody conjugation technology has also undergone three generations of development, from the non-specific coupling of the first generation, to the site-specific coupling of the second generation through genetic engineering, and finally to the third generation that does not rely on genetic engineering for site-specific coupling. This article will provide a comprehensive analysis of the three generations of conjugation technology and focus on the site-specific coupling technology that does not rely on genetic engineering.
1
First Generation Antibody Conjugation Technology
The concept of combining the specificity of antibodies with the toxicity of drugs to create a higher level of targeted therapy can be traced back to 1913, when Paul Ehrlich proposed the development of a “magic bullet” for selectively targeting tumors. Despite the simplicity and novelty of this concept, the first ADC was not used clinically until 2000, when the FDA approved Gemtuzumab ozogamicin (Mylotarg, Pfizer/Wyeth) for the treatment of acute myeloid leukemia. In 2011, Brentuximab vedotin (Adcetris, Seattle Genetics) was approved for the treatment of anaplastic large cell lymphoma. Two years later, Trastuzumab emtansine (Kadcyla, Genentech/Roche) was approved for the treatment of advanced HER2-positive breast cancer. To date, there have been 14 ADCs approved, including Inotuzumab ozogamicin; Polatuzumab vedotin; Enfortumab vedotin; Trastuzumab deruxtecan; Sacituzumab govitecan; Belantamab mafodotin; Moxetumomab pasudotox; and Loncastuximab tesirine.
In most of these cases, the ADCs were completed using first-generation conjugation technology. These technologies utilized reduced cysteine residues (four pairs of interchain disulfides in IgG1) or lysine residues exposed on the surface of the antibody (approximately 80 potential coupling sites). First-generation antibody conjugation methods utilized N-hydroxysuccinimide (NHS) or maleimide-mediated crosslinking to stably attach a functional molecule to the primary amine of lysine or the thiol group of cysteine. Due to the large number of potential reaction sites, these conjugation methods inevitably resulted in ADCs with different drug-antibody ratios (DAR), leading to significant product heterogeneity. Current research shows that these heterogeneous drugs have suboptimal therapeutic effects compared to drugs with stronger uniformity.
2
Second Generation Antibody Conjugation Technology
2.1 Additional Introduction of Cysteine
The first systematic proof that site-specific conjugated drugs have better efficacy came from Genentech’s Junutula and colleagues, who utilized a site-specific conjugation technology called THIOMAB. This technology primarily involved the introduction of an additional cysteine, which under reducing conditions, the introduced cysteine and the interchain disulfides were reduced, oxidized under the action of CuSO4 to reform disulfide bonds, and the drug was ultimately conjugated to the additional introduced cysteine through a maleimide chemical reaction. Compared to the first-generation conjugates with an average DAR of 3, the DAR of THIOMAB’s site-specific conjugate ADCs was 2. Researchers used this technology to couple MMAE with an anti-MUC16 antibody and achieved better efficacy in ovarian cancer animal models. More importantly, this drug not only improved efficacy but also had higher dose tolerance and increased stability in rat and monkey serum.
THIOMAB™ Site-Specific Conjugation Platform
2.2 Introduction of Non-Natural Amino Acids
Due to the growth of the ADC market and the advantages of site-specific conjugation technology, more researchers have focused on developing ADC drugs using second-generation antibody conjugation technology. Generally, second-generation antibody conjugation technology requires the introduction of a reactive group with a unique site specificity into the antibody, followed by selective conjugation of a functional molecule with this unique group using bioorthogonal reactions. Currently, strategies for introducing non-canonical amino acids (ncAAs) into proteins have been employed in both prokaryotic and eukaryotic cells, providing a relatively straightforward approach for site-specific conjugation. A bioorthogonal ncAA—p-acetylphenylalanine (pAcF) was first used to explore site-specific conjugation. Researchers used an acyl-tRNA synthetase (aaRS)/tRNA pair that is orthogonal to the host’s endogenous translation machinery to site-specifically insert pAcF at the amber codon of trastuzumab. Using this method, researchers obtained trastuzumab containing double pAcF (one on each heavy chain). It was then coupled with an alkoxy amine-derived drug monomethyl auristatin E to ultimately yield an ADC with a DAR of 2, which exhibited the same efficacy as first-generation ADCs but with improved safety.

pAcF Site-Specific Conjugation
2.3 Introduction of Specific Tags
Although site-specific conjugation can be achieved by introducing non-natural amino acids, the expression of antibodies has been greatly limited in production. To avoid this issue, some researchers have introduced short peptides and utilized enzyme-catalyzed reactions for site-specific conjugation. The aldehyde tag-based SMARTag short peptide sequence is CXPXR, which can be site-specifically inserted into a specific location of the antibody through genetic engineering. The cysteine in the tag can be oxidized to form formylglycine under the action of formylglycine-generating enzyme (FGE). Scientists at Redwood Bioscience found that antibodies containing formylglycine sites could undergo site-specific conjugation using bioorthogonal reactions, with oxime linkages and alkoxy amine and hydrazine-Pictet-Spengler (HIPS) linkages being two examples of such conjugations. Using this technology, relevant antibodies developed by Redwood Bioscience have entered clinical trials.

SMARTag™ Platform
2.4 Enzyme-Catalyzed Reactions
Utilizing enzyme-catalyzed reactions for covalent coupling, researchers have employed glutamine transferase and sortase for site-specific coupling of antibodies. Glutamine transferase can catalyze the formation of amide bonds between glutamine and primary amines. The Schibli team was the first to utilize bacterial glutamine transferase for site-specific coupling, but their research found that although there are many glutamines in the antibody, the enzyme-catalyzed reaction only occurred at Gln295 after removing the glycosylation at N297 or performing an N297S mutation. Other researchers introduced a short peptide tag LLQGA containing glutamine, which under the catalysis of glutamine transferase, formed ADCs with DAR values of 1.8-1.9.

Using a similar approach, sortase can also catalyze reactions on specific amino acid sequences, linking poly-glycine and the LPXTG short peptide tag, allowing antibodies to be site-specifically conjugated by genetically engineering the LPXTG short peptide tag.

2.5 Introduction of π-Clamps
In addition to utilizing enzyme-catalyzed reactions, some studies have introduced specific amino acid sequences to the end of the antibody heavy chain, such as FCPF, which contains four amino acids that provide a special environment conducive to site-specific modification of cysteine (for peptides containing the “π-clamp” sequence, when part of the “π-clamp” undergoes a gly mutation and then reacts under competitive conditions, only those peptides containing the “π-clamp” sequence will undergo quantitative reactions).
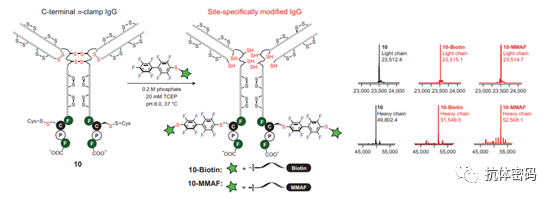
Summary: Overall, second-generation site-specific conjugation technology is achieved by introducing bioorthogonal molecules into antibodies and then utilizing the introduced unique sites for site-specific conjugation. However, the introduction of these unique sites often requires modification of the antibody, which may alter some of the antibody’s properties and even affect its folding or stability, thus posing certain challenges for subsequent generation studies.
3
Third Generation Antibody Conjugation Technology
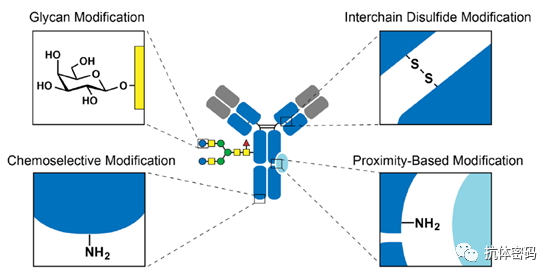
Although second-generation antibody conjugation technology can achieve site-specific conjugation, it requires special modifications to the antibody, which may pose new challenges for the development of ADC drugs. Therefore, some researchers have begun to consider site-specific coupling of natural antibodies without altering the integrity of the antibody. The third-generation conjugation technology mainly focuses on selecting unique amino acid sites and utilizing unique molecules or proximity effects for site-specific coupling. It is primarily divided into the following four types: 1) Interchain disulfide bond modification; 2) Glycosylation modification; 3) Chemical selective modification; 4) Proximity effect-based modification.
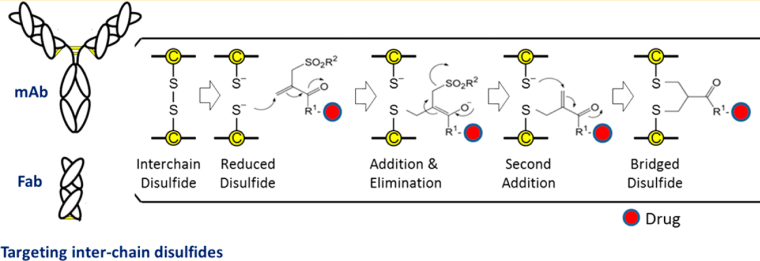
3.1 Interchain Disulfide Bond Site-Specific Conjugation
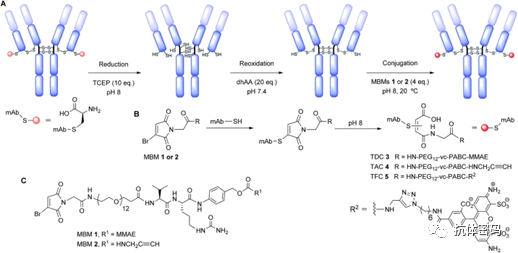
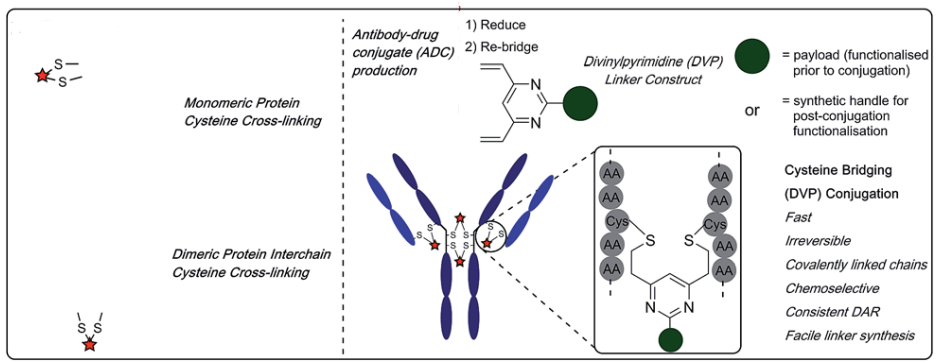
Coupling the payload to the antibody using cysteine requires a reaction between the reduced thiol groups in the antibody and the electrophilic species in the payload. Compared to other lysine residue-based coupling methods, this method has certain advantages because cysteine is less abundant in antibodies, which allows for higher specificity in coupling. The interchain four disulfide bonds can be reduced to eight free thiols, allowing for the highest possible uniform ADC with a DAR value of eight. The disulfide reconnecting technique was developed by the Smith and Lawton research team in 1990, and this method allows for site-specific coupling without affecting the natural structure of the antibody. It mainly consists of the following four steps: (1) Reduction of disulfide bonds to obtain two active cysteines; (2) Reaction of the reconnecting reagent in the payload with one of the cysteines; (3) The remaining cysteine reacts with the cysteine-reconnecting reagent complex generated in the previous step to form a new linkage.
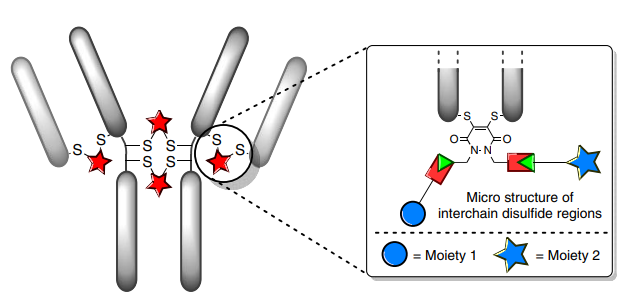
Depending on the coupling reagents used, interchain disulfide bond site-specific conjugation can be divided into four categories (see below): a: mono/di-sulfide reagents; b: 3,4-disubstituted maleimides; c: dibromodiphenyl ketone; and d: divinyl pyridine. However, regardless of the reagent used, it is necessary to reduce the interchain disulfide bonds (there are 16 disulfide bonds in IgG antibodies, but four interchain disulfide bonds are more susceptible to solvent exposure and can be reduced) using reducing agents such as DTT, TCEP, or 2-mercaptoethanol, where DTT and 2-mercaptoethanol require a neutral or alkaline environment, so they must be removed after the reaction, or they may react with the reconnecting reagents in subsequent steps, affecting coupling efficiency. TCEP, on the other hand, can effectively reduce disulfide bonds in the pH range of 1.5 < pH < 8.5, so it does not need to be removed in subsequent steps.

3.1.1 Mono/Di-Sulfide Reagents
For di-sulfide reagents, the first step is to activate by removing one sulfonate to obtain a mono-sulfide, which then reacts with a thiol in the antibody to form a disulfide bond, while the remaining sulfonate is removed to obtain a Michael receptor that reacts with another thiol in the antibody, thereby linking the disulfide bonds in the antibody. In 2014, Badescu et al. utilized this approach to couple the Trastuzumab antibody, obtaining an ADC containing MMAE with a DAR value of 4, and it also exhibited high stability in a 10 mM DTT reduction environment (see above image a1). Subsequently, researchers have used alkylated intermediates of di-sulfide reagents for coupling, as these reagents have higher solubility, resulting in higher reaction efficiency (see above image a2).
Mono-Sulfide Coupling
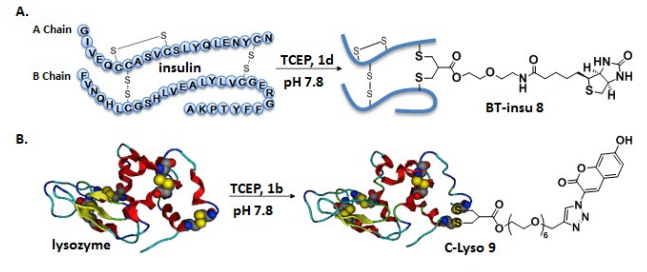
Di-Sulfide Reagent Alkylation Coupling
3.1.2 3,4-Disubstituted Maleimides
3,4-Disubstituted maleimide coupling occurs with second-generation maleimide reagents (NGMs) such as 3,4-dihalo maleimides and disulfide maleimides. Compared to mono/di-sulfide reagents, the reaction pH is broader (6.2-8) and the reaction speed is fast, effectively reducing the aggregation of antibodies during the reaction (see below image b). Additionally, the DAR value can be achieved from 0-4 by controlling the dosage of the reaction reagents and reaction time.
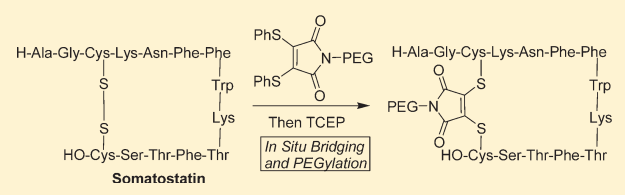
3.1.2 Dibromodiphenyl Ketone
Similar to the reaction mechanism of 3,4-disubstituted maleimides, some researchers have utilized dibromodiphenyl ketone for coupling. The two nitrogen atoms in the dibromodiphenyl ketone ring provide potential for the coupling reaction, achieving a one-step coupling that reduces the influence of the coupling reagents on the antibody (see above image c).
3.1.2 Divinyl Pyridine
In 2019, Walsh et al. utilized divinyl pyridine containing two Michael receptors to react with the reduced disulfide bonds, achieving a high coupling yield. However, due to the long chain of the reagent bridging the disulfide bonds (spanning seven carbon atoms), there was some disturbance from disulfides during the reaction.
Summary: Currently, many studies have utilized mono/di-sulfides, NGMs, divinyl pyridine, etc., for specific coupling with the disulfide bonds of natural antibodies. However, there are still many limitations and pitfalls. Firstly, the disturbance of disulfides during the coupling process is unavoidable, which can affect the efficacy, yield, and scalability of the product. Although some methods have been proposed to improve this issue, such as precise control of reaction conditions, increasing the kinetics of bridging, and using TCEP-linked phenylthio derivatives for integrated reactions, these need to be optimized based on the specifics of different drugs. Secondly, under reducing conditions, these bridging reagents cannot distinguish between reduced disulfides and favorable thiols, which limits the bridging of disulfides that lack free cysteines in antibodies. Wilson et al. reported a trivalent arsenic (As(III)) acid derivative as a potential solution for the generated orthogonality of disulfide thiols since the mono-thiol arsenic acid complex is entropically unfavorable. However, this usage has not been demonstrated in antibody applications, and the issue of in vivo toxicity concentrations related to therapeutic antibodies still needs to be resolved. Lastly, the poor solubility of bridging reagents often requires changing the ratio of co-solvents to facilitate reactions, increasing the risk of antibody denaturation. If using di-sulfide reagents, this issue can be alleviated by using water-soluble mono-sulfide intermediates, while for other reagents, it can be solved by additionally introducing PEG linkers.
3.2 Glycosylation Coupling
There are two conserved glycosylation sites N297 in IgG-type antibodies, which are located in the CH2 domain, away from the functional domain that binds the antibody to the antigen. Therefore, utilizing these glycosylation sites for coupling will not affect the binding function of the antibody. Overall, the coupling based on glycosylation mainly involves introducing a new reactive group on the glycan, followed by site-specific coupling using related reagents.

3.2.1 Glycan-Oxidation Based Modifications
Oxidative cleavage of terminal cis-diol groups in oligosaccharides generates an aldehyde group, and some reagents containing aminooxy, hydrazine, or acylhydrazine functional groups can chemically react with it, allowing for the site-specific conjugation of the payload to the antibody. O’Shannessy and colleagues were the first to oxidize the glycan of the antibody using periodate to form aldehyde groups, which were then reacted with hydrazine-labeled biotin. Similar methods have been used for the conjugation of various molecules, including radiolabeled organometallic complexes, toxins, and proteins/antibodies. Although these methods indeed achieve site-specific conjugation of natural antibodies, the heterogeneity of antibody glycosylation greatly limits the applicability of this method. Additionally, high concentrations of periodate (10-30 mM) can oxidize sensitive methionine residues, potentially harming the antibody’s serum half-life, integrity, and efficacy.
To avoid this problem, researchers introduced periodate-sensitive sialic acid into the N-glycans of antibodies (see below image). To achieve specific modifications, galactose and sialic acid residues were enzymatically incorporated into the oligosaccharides of natural antibodies using β1,4-galactosyltransferase (Gal T) and α2,6-sialyltransferase (Sial T). MALDI-TOF analysis showed that over 94% of the N-glycan residues were converted to single sialylated sugars. Using 1 mM periodate to oxidize the sialic acid residues and generate aldehyde groups, they were coupled with aminooxy-functionalized cytotoxins, yielding an average DAR of 1.6. Although these ADCs exhibited significant anti-tumor efficacy both in vitro and in vivo, low concentrations of periodate still somewhat affected the binding of FcRn to the antibody.

3.2.2 Glycan-Non-Oxidative Based Modifications
To completely avoid periodate treatment, some research groups have performed enzymatic modifications on oligosaccharides by introducing sugar residues containing bioorthogonal groups. Bogman et al. showed that using a β1,4-galactosyltransferase mutant GalT (Y289L) could incorporate galactose containing a keto handle at the C2 position into the antibody oligosaccharides. Selective coupling could then occur between the keto handle and derivatives containing aminooxy (biotin or fluorescent dyes). However, the conversion rate and efficiency of this process need further improvement. Zeglis et al. applied enzyme-mediated glycoengineering and strain-promoted azide-alkyne cycloaddition reactions to achieve site-specific radiolabeling of PSMA antibody J591’s modified oligosaccharides (see below image). They used β1,4-galactosidase to remove terminal galactose, then used GalT (Y289L) to introduce azide-modified galactose as the terminal residue of the glycan. Then, using a chelator containing desferrioxamine (DFO), they employed strain-promoted azide-alkyne cycloaddition to connect it to the antibody. Finally, mixing the chelator-modified antibody with 89Zr generated the 89Zr-labeled ADC. The 89Zr-labeled antibodies produced by this method were selectively absorbed by tumors and exhibited excellent anti-tumor activity in a mouse model of prostate tumors expressing PSMA.

As an alternative strategy, Li and colleagues used Gal T to remodel the antibody glycosylation, transforming the existing glycoform into G2 glycoform (see image E). They then incorporated sialic acid derivatives containing azide at the C9 position into the N-glycans using sialyltransferase ST6GalI. Subsequently, dibenzylcyclooctyne (DIBO)-modified biotin, fluorescent dyes, or cytotoxic drugs reacted with the azide-containing antibodies, resulting in antibody conjugates with DAR values of 3.5-4.5. This glycosylation remodeling strategy provides an attractive alternative for preparing homogenous ADCs with unchanged FcγRIIIa binding. For pharmacokinetic reasons, to achieve a lower DAR, van Delft and colleagues reported a novel chemical enzyme coupling strategy to generate ADCs with a DAR of 2 (see image F). After deglycosylation and separately introducing GalNAz via endo-S2 glycosidase and galactosyltransferase GalT (Y289L), the modified antibodies could be linked to the effective payload through copper-free click chemistry, producing highly stable and homogeneous ADCs with a DAR of approximately 2.
3.2.3 Metabolic Engineering of Antibody Oligosaccharides
In addition to using enzymatic and chemical strategies for engineering antibody oligosaccharides in vitro, ADCs can also be obtained by introducing glycan analogs containing bioorthogonal reactive groups through metabolic engineering of the oligosaccharide residues. Okeley and colleagues first reported the use of fucosyltransferase VIII to introduce fucose analog 6-thiofucose at the N-glycan terminus (see below image). Specifically, 6-thiofucose, screened from approximately 200 synthetic fucose analogs, can replace fucose in the antibody oligosaccharides with an efficiency of 60-70% through peracetic acid. Subsequently, 6-thiofucose was coupled with MMAE through maleimide, resulting in an ADC with an average DAR of 1.3. Compared to ADCs prepared by interchain disulfide/maleimide coupling chemistry, ADCs obtained using this strategy maintained improved plasma stability and anti-tumor activity in vitro.

Summary: Although significant progress has been made in obtaining IgG with bioorthogonal glycoforms, most enzyme-mediated glycosylation engineering techniques remain in the exploratory stage at the laboratory scale. Moreover, producing ADCs always requires introducing some non-natural components into the antibody glycan. Some subtly modified glycans have been reported to have immunogenicity in humans. Therefore, the in vivo stability and immunogenicity of glycoengineered antibody conjugates still need to be fully assessed.
3.3 Chemical Selective Modifications
Conceptually, performing chemical reactions based on chemical selectivity to directly modify specific amino acid residues is the most straightforward method for modifying natural antibodies. However, slight differences in reactivity among nucleophilic amino acids make it challenging to achieve the desired chemical modification specificity. Additionally, due to residue heterogeneity among structurally similar antibodies, chemical and regioselective modification methods are often only effective in specific cases, limiting the generalizability of this approach. A widely used concept is to target lysines with the lowest pKa by precisely controlling the reaction pH; for example, the basicity of N-terminal amines is weaker than that of other lysines, allowing them to be targeted under pH 7.7 ±0.5 conditions. The latest developments in site-specific antibody modifications based on chemical selectivity are summarized below.
3.3.1 N-terminal Transamination
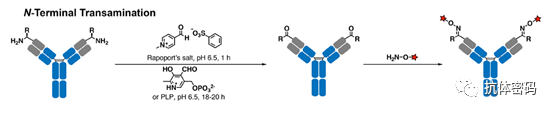
In 2007, Scheck et al. reported the use of pyridoxal-5′-phosphate (PLP, vitamin B6) to transform the N-terminal amine of immunoglobulin light chains into ketones through an amine transfer reaction. The ketones can then be easily functionalized through oxime linkage. The basicity of the N-terminal amine (imine formation) and the more acidic N-terminal α-proton (tautomerization) is the basis for the chemical selectivity of N-terminal amines compared to other lysine side chains. Moderate conversion rates (47%) were achieved at elevated temperatures (50°C). Based on the same transamination mechanism, Witus et al. selectively transformed the N-terminal amine of immunoglobulin heavy chains into ketones using Rapoport salt (RS) under much milder conditions (37°C), increasing the yield to 67% (see below image). These amine transfer reagents exhibited some residual preferences, such as PLP preferring light chain aspartate over heavy chain glutamine in mouse IgG, while RS showed a preference for heavy chain glutamate over light chain aspartate in human IgG. Further experiments demonstrated that the optimal reaction partners for PLP and RS were the tripeptides H-Ala-Lys-Thr and H-Glu-Glu-Ser, respectively. Since these N-terminal sequences do not exist in natural antibodies, genetic engineering is required to obtain optimal reactivity.
3.3.2 Lysine-Specific Reagents
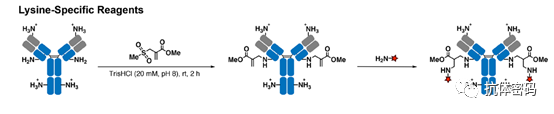
Several attempts have been made to selectively modify certain lysine residues in antibodies based on controlling reaction kinetics, protein site specificity, or targeting highly reactive lysines. However, due to the unclear mechanisms of lysine selectivity, these methods have not achieved high yields. Therefore, it is challenging to apply them to the modification of antibodies or other proteins. To address this issue, Matos et al. reported the use of acrylamide sulfonate-based reagents, combined with pH control, to achieve selective and high-yield (>95%) modification of a single lysine with the lowest pKa (see below image). The sulfonyl oxygen stabilized the positive charge generated by the amine through a chair-like transition state addition. The relatively polar cysteine S-H groups reduced hydrogen bond interactions, explaining the chemical selectivity of lysine over cysteine. Regioselectivity is primarily based on pKa control, as lysines with the lowest pKa are most likely to be reactive under neutral to slightly alkaline conditions. Lysine residues modified with acrylamide can further react with amine-containing compounds via nitrogen-centered Michael addition. The universality of this modification strategy has been successfully demonstrated with five model proteins, including a humanized full-length trastuzumab antibody. Flow cytometry experiments showed that the site-specific modification of trastuzumab with the anticancer drug crizotinib did not affect its binding affinity and specificity for human HER2. However, if the modified lysine is critical to the antibody’s function, the conjugation may fail.
3.3.3 Key Point-Oriented Modifications
Adusumalli et al. developed another reactivity-based method called key-directed modification (LDM). LDM utilizes fast and reversible amine reactive groups to guide slow irreversible reactions between epoxide groups and histidine residues in proteins (see below image). While a fast switch balance was established between hydroxyl benzaldehyde and lysine residues, the linked epoxide portion would only react with histidine residues located at a certain distance from the lysine residues. After the irreversible connection between histidine and the epoxide, the free aldehyde can undergo oxime linkage to conjugate other desired molecules. Two ADCs, trastuzumab-doxorubicin and trastuzumab-emtansine, have been produced using this technology and exhibited anti-tumor activity in vitro. To broaden the substrate range of LDM, Adusumalli et al. reported another LDM reagent for modifying lysine rather than histidine. The conversion of the leaving group from the epoxide to 2,6-dibromo-4-(morpholine-4-carbonyl)phenyl ester provided more options for optimizing substrates.
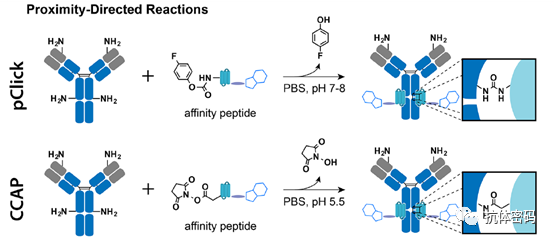
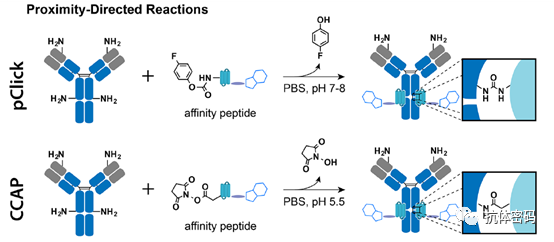
Summary: Selective reactions between probes and specific natural residues of proteins (e.g., lysine, cysteine, serine) using proximity coupling methods are becoming an important class of antibody conjugation methods. These strategies enable the preparation of site-specific antibody conjugates without the need for additional antibody engineering or modifications. Currently, efforts based on proximity coupling are mainly focused on new proximity-inducing chemistries and antibody binding agents that cause little disruption to antibody structure and function.
4
Conclusion
Due to the high therapeutic index and excellent biochemical properties of site-specific ADCs, many efforts are currently focused on antibody site-specific conjugation strategies. Multiple methods have been developed for the specific covalent connection of functional molecules to antibodies, most of which require antibody engineering to install unique reactive portions at specific sites, followed by selective modification of these portions using bioorthogonal chemistry. While these techniques allow for excellent control over conjugation sites, they have technical challenges and a lack of generalizability in antibody engineering strategies. Therefore, methods for site-specific conjugation of natural antibodies are more attractive.
The core of precise labeling of natural antibodies is the ability to efficiently perform site-selective modifications on individual desired amino acid residues without generating off-target effects. With advances in bioorthogonal chemistry, enzyme engineering, and proximity chemistry, various methods have emerged, such as targeting unique interchain disulfides, glycans, and N-terminal residues, as well as using proximity effects to guide functional probes’ reactions. Despite many options for the specific modification of natural antibodies, these strategies still face certain limitations in some applications: disulfide rebridging may be affected by disulfide disturbance and batch-to-batch variations; the different forms of IgG glycosylation and the high valency of glycoanalogues significantly limit the efficiency and scalability of antibody glycan-based labeling methods; chemical selectivity and proximity-based antibody modifications require fine-tuning of chemical reactivity. As research progresses, the continuous development of protein engineering and protein chemistry combined with new design concepts may allow for the production of natural antibody conjugates with excellent biochemical properties and therapeutic effects without complex chemistry or enzymatic treatments in the future.
References
Service: This public account accepts free submissions of research progress, R&D stories, etc. from research teams/units for non-commercial/non-profit purposes, as well as free publication of recruitment advertisements for research teams, etc. Welcome to submit.
Statement: The publication/reprinting of this article is solely for the purpose of disseminating information and does not imply the views of this public account or confirm the authenticity of its content. Any judgment made based on this content is at your own risk.If there is any infringement, please inform us and we will delete it!
Long press to follow this public account

