GUIDE
Editor’s Recommendation
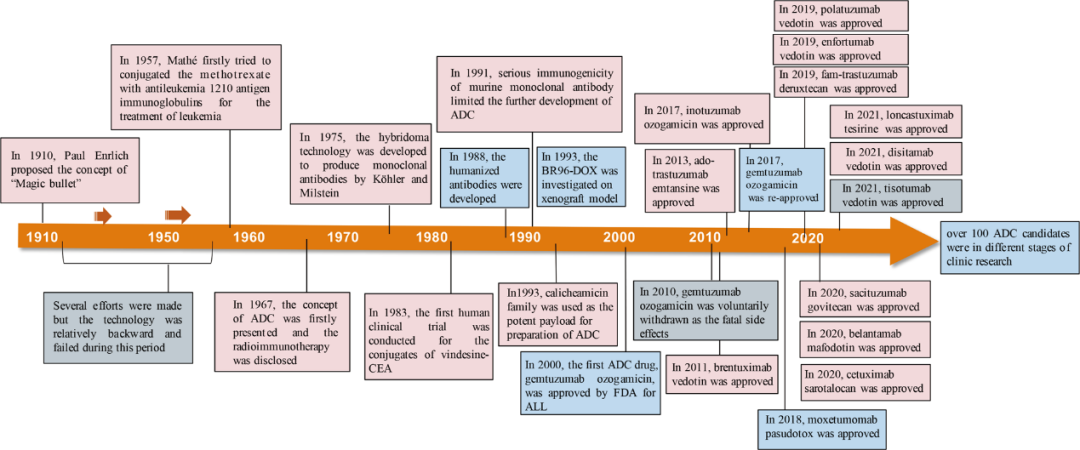
Antibody-drug conjugates (ADC) remained popular in 2022. Enhertu redefined the treatment landscape for HER2 breast cancer, making headlines at major academic conferences like ASCO and ESMO; Kelun-Biotech approached a $10 billion license-out deal with multinational pharmaceutical company Merck, setting a new record for local pharmaceutical companies going abroad; the world’s first anti-CD79b ADC was also approved in early 2023 in China, rewriting the treatment standards for diffuse large B-cell lymphoma that have stood for 20 years.
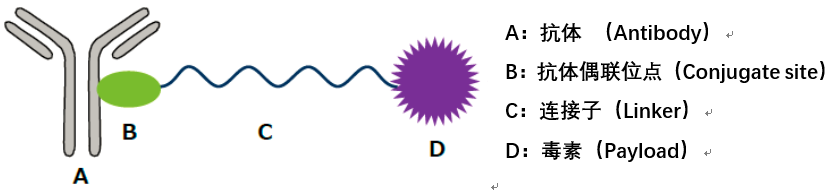
ADC consists of three components: antibody, toxin (payload), and linker. From a technical perspective, while the antibody is generally an existing product on the market, there is considerable room for innovation in the toxin and linker components, leading to the emergence of the widely recognized first, second, third, and fourth generation ADC technologies. The advantages and disadvantages of each generation of ADC technology and areas for further optimization may be a matter of perspective. Merck has specifically targeted Kelun-Biotech’s linker-payload technology and plans to package and introduce ADC projects using this linker-payload.
Dr. Song Shuai joined Kelun-Biotech for innovative drug research after graduating with a PhD in 2015, and over the past seven years, he has led nearly ten molecules into different clinical stages, including some ADC linker-payloads transferred to Merck, which he was responsible for. Recently, PharmaCube invited Dr. Song to share his technical insights on payload and linker for everyone’s reference.

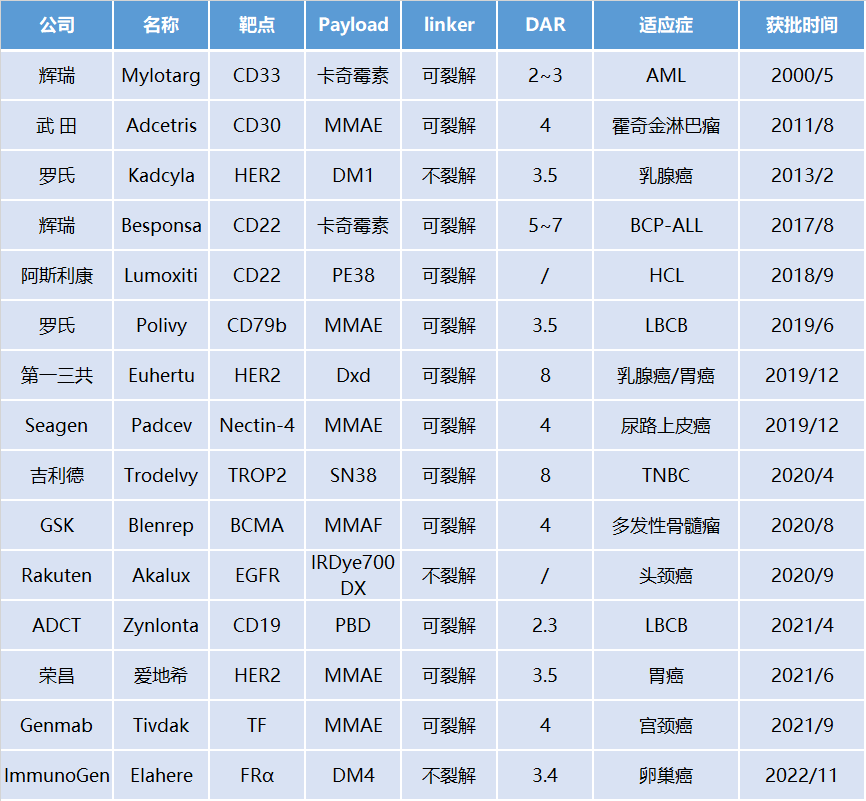
Approved ADC Drugs Worldwide
-
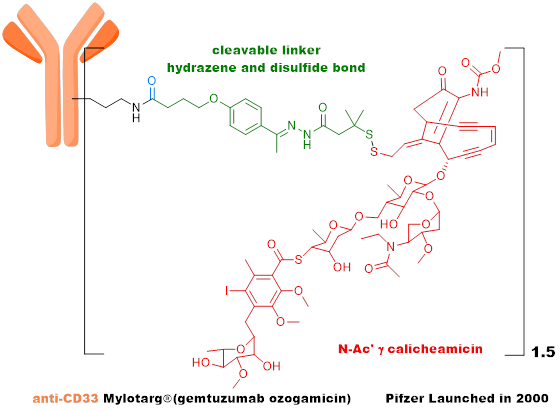
The first generation ADC uses mouse-derived antibodies + highly active payload + unstable linker + random conjugation strategy, with high immunogenicity, consisting of a mixture of different DAR value components, unstable metabolism, narrow therapeutic window, and high challenges in drug development, with Mylotarg as a representative product. -
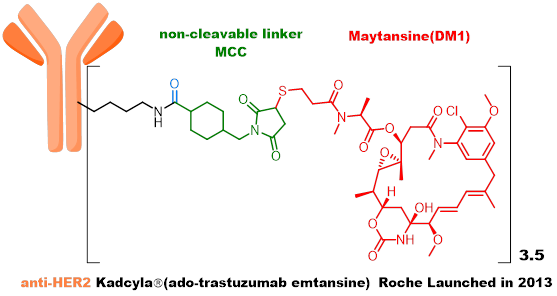
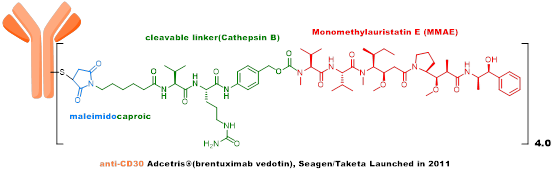
The second generation ADC uses humanized antibodies + highly active payload + stable linker + random conjugation strategy, with low immunogenicity, significantly improved metabolic stability, and increased therapeutic index, with Kadcyla and Adcetris as representative products. -
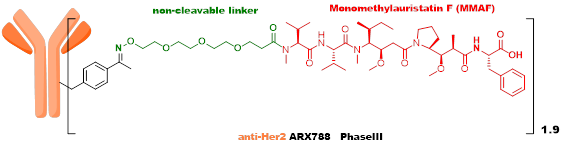
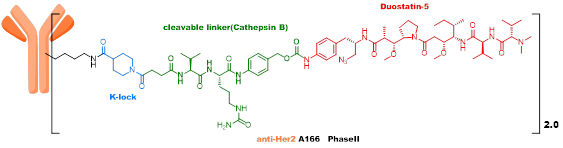
The third generation ADC employs site-specific quantitative conjugation technology, using low DAR values and highly active payload strategies, with good metabolic stability and uniformity, showing promising initial clinical results that still need further verification, with ARX788 and Sichuan Kelun’s A166 as representative products. -
The fourth generation ADC technology uses high DAR values (4-8) and a medium-low activity payload strategy, with the payload having a high bystander effect. The most important feature is that it has good efficacy against tumors with both low and high expression of surface antigens, with Enhertu, Trodelvy, and Sichuan Kelun’s SKB264 as representative products.
The third generation ADC learned from the experiences of early ADCs, recognizing that heterogeneity and instability are important factors affecting the in vivo efficacy and toxicity of ADCs. High DAR value components have poor stability in vivo, affecting the therapeutic window of ADCs, so a strategy of site-specific conjugation and low DAR values was adopted. ARX788, developed by Ambrx, is currently in Phase III clinical trials, using an MMAF analog as the toxin, with a non-cleavable linker connected through unnatural amino acids on the antibody. A166, developed by Sichuan Kelun, is currently in Phase II clinical trials, also using an MMAF analog as the toxin, with a VC linker connected through K-Lock technology to the antibody.
This generation of ADC uses new generation site-specific conjugation technology, with high uniformity and good stability. Representative technologies include: 1) engineered cysteine; 2) unnatural amino acids; 3) enzyme-catalyzed conjugation; 4) glycosylation conjugation; 5) special conjugation linkers. Due to the lower DAR value, the payload tends to be more active toxin molecules (such as PBD, Tubulysin, etc.).
Through analysis of existing products, we find that site-specific conjugation is not the key factor affecting ADC toxicity; rather, the core issue is whether the products obtained through site-specific conjugation have better stability.
The fourth generation ADC has three representative products (Enhertu, Trodelvy, and SKB264) that show significant efficacy and have enormous potential for future innovation. Enhertu has redefined the treatment of breast cancer and is challenging first-line therapies; Trodelvy is the first ADC drug targeting triple-negative breast cancer and has achieved $21 billion in transaction volume; SKB264 shows clear clinical effects, and the related technology platform has achieved substantial transactions with MNCs.
Analyzing these three products, the payload’s mechanism of action is novel, and there have been significant updates in the linker aspect. All payloads use camptothecin-based topoisomerase I inhibitors, which have the following four characteristics: ① Targeting DNA with low “concentration” of the target; ② Low toxicity, high plasma clearance rate, and relatively low systemic toxicity; ③ High permeability with a good bystander effect; ④ Effective against tumors resistant to microtubule inhibitors.
In terms of linkers, Enhertu uses a novel four-peptide (GGFG) + self-cleaving fragment + maleimide linker, where GGFG can be cleaved by various tissue proteases (Cathepsin). The new self-cleaving fragment was developed for the first time by Daiichi Sankyo, and the conjugation uses the conventional maleimide linker, which can fall off in systemic circulation, leading to systemic toxicity.
Trodelvy employs an innovative “self-cleaving” linker that can release toxins in pure water, blood, and the tumor microenvironment, thus achieving antitumor effects without requiring intracellular endocytosis. However, due to the weak activity of the payload used, and its poor stability in systemic circulation, there is still significant room for improvement in efficacy, and its indications cannot be further expanded.
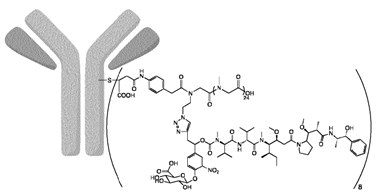
SKB264 uses an innovative pyrimidine conjugation linker, which significantly enhances metabolic stability compared to maleimide linkers, while the payload uses a new camptothecin compound with high activity. SKB264 improves the chemical stability of ADC molecules by modulating the physicochemical properties of both overall and local ADC molecules, resulting in a significantly longer half-life in pure water and plasma compared to Trodelvy, and showing significantly better efficacy in preclinical animal models, with a markedly improved therapeutic index. SKB264 is currently in Phase III clinical trials. The payload-linker technology of SKB264, based on existing patents, shows the potential for application in the treatment of various tumors.
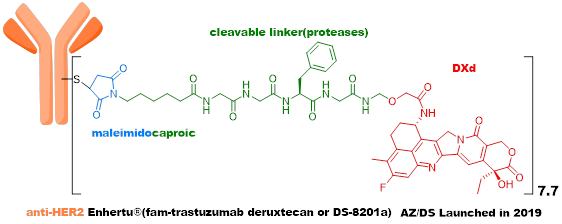
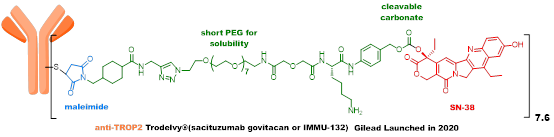
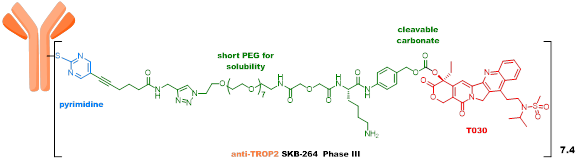

Although the new generation ADC represented by Enhertu has achieved great success, there is still significant room for improvement.
-
From the perspective of payload, the mechanism of action is relatively singular (microtubules, DNA), mainly consisting of cytotoxic compounds, with limited selection, often leading to resistance. -
From the perspective of linker, traditional ADCs require processes such as tumor cell endocytosis and lysosomal cleavage, and many steps can cause ADC resistance or complete lack of therapeutic effect. Additionally, traditional endocytosis mechanism ADCs perform better for high-expression HER2 ADCs but relatively poorly for other low-expression target ADCs; linkers have insufficient stability in the circulatory system, easily causing systemic toxic reactions. -
From the perspective of ADCs, they have a large molecular weight, slow tumor accumulation rate, low permeability, and traditional techniques require antibodies to be endocytosed to be effective, limiting antibody selection for ADC development.
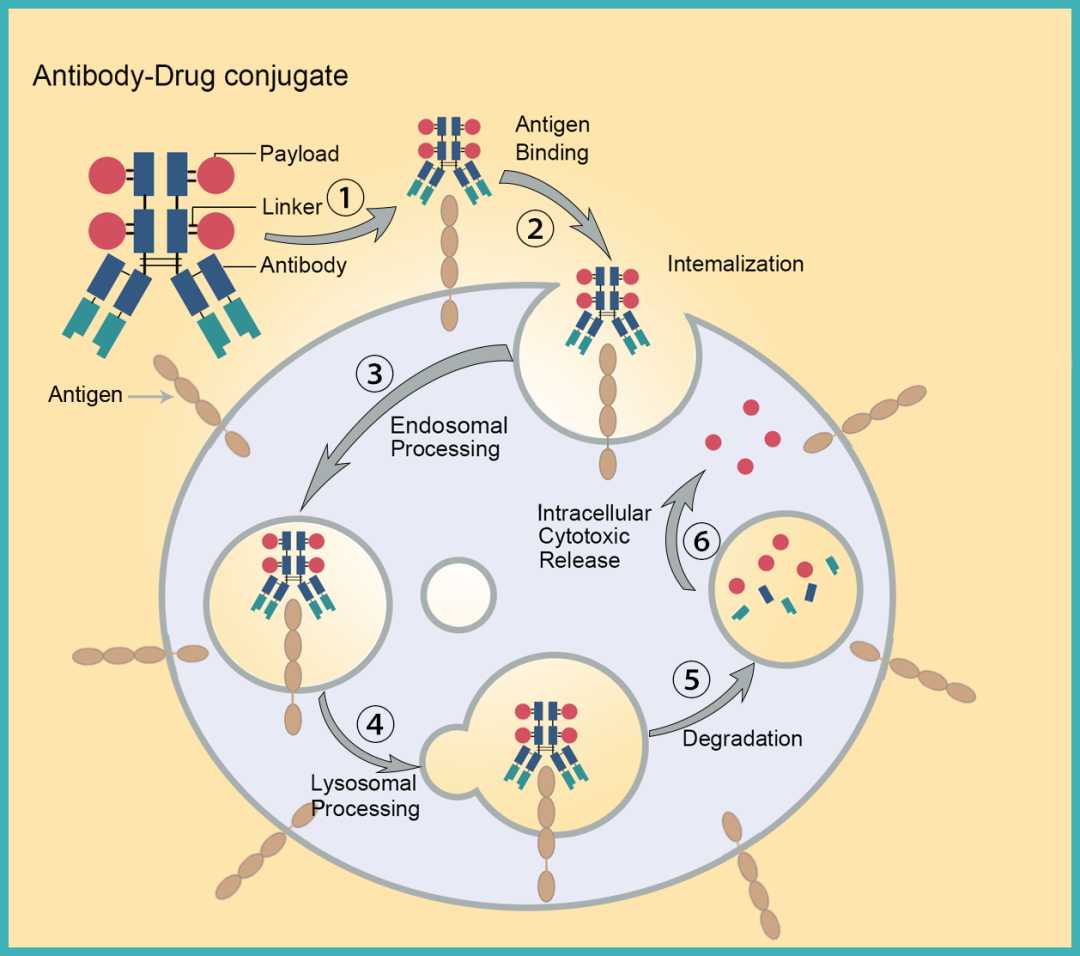
Traditional ADC Mechanism of Action

Regarding innovative directions for next-generation ADCs, in general, the approach is to address the shortcomings of existing ADC technologies by further optimizing payload and linker, enhancing ADC stability, increasing toxin “utilization,” and expanding new toxins to improve ADC efficacy and overcome resistance.
Exploring new mechanisms of action from the perspective of payload.That is, guided by clinical needs, the payload shifts from cytotoxic drugs to other targets. For example, the following directions:
-
First, tumor immune agonists, involving targets such as TLR, STING, etc. Since these molecules may activate systemic non-specific immune responses, they can lead to life-threatening cytokine storms in severe cases, making it difficult for small molecules to become drugs. As ADC payloads, they can form ISAC with antibodies, activating both innate and adaptive immunity, transforming “cold tumors” into “hot tumors” to exert stronger immune effects. Numerous companies, including Bolt, Silverback, Tallac, Takeda, Mersana, Seagen, Hengrui, Qide, and Jaco, have already engaged in research on ADCs with TLR agonists and STING agonists as payloads. The highest progress for TLR-type ADCs has reached Phase I clinical trials, but no substantial progress has been seen yet; STING-type ADCs are still in the preclinical stage, and whether ADCs can overcome the efficacy issues of STING small molecules in clinical settings is highly anticipated.
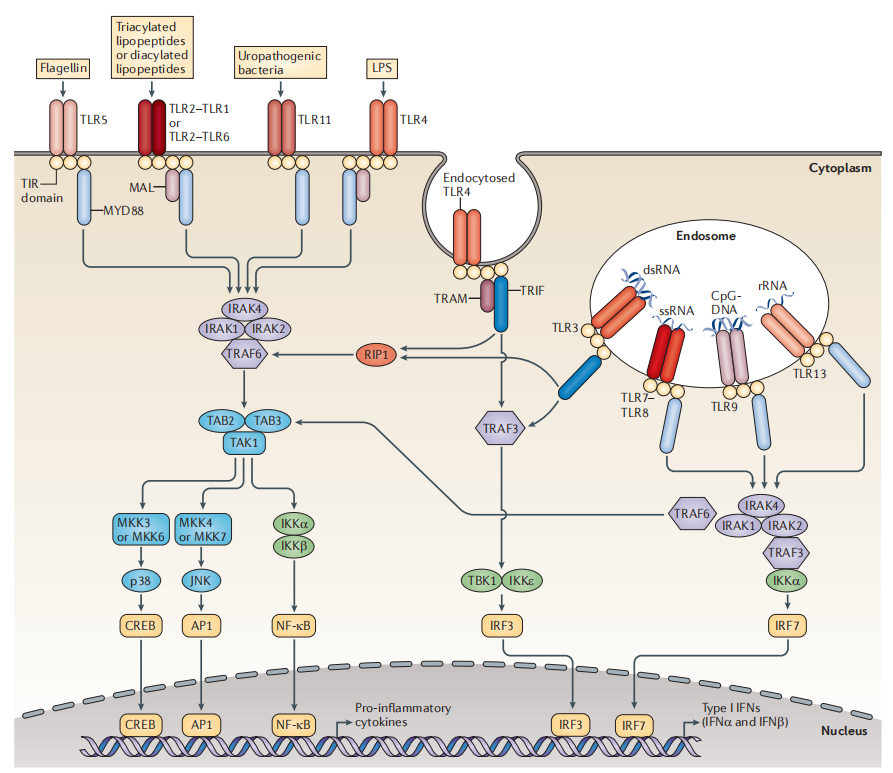
TLR Immune Agonist Signaling Pathway
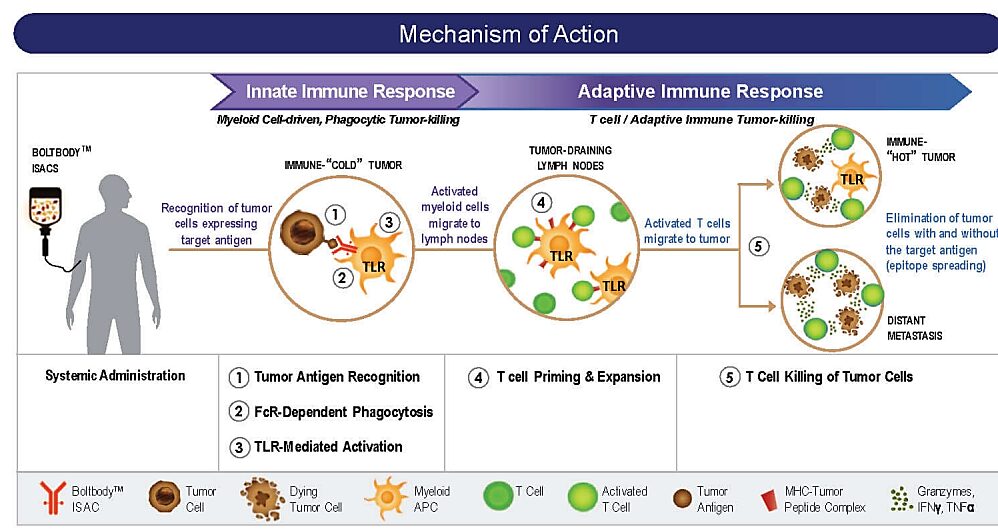
MOA of TLR ADC (Source: Bolt Biotherapeutics)
-
Second, protein degraders, including PROTAC and molecular glues. The main characteristic of these molecules from a mechanistic perspective is that they can produce strong efficacy with a “catalytic amount.” PROTAC molecules include E3 ubiquitin ligase ligands, target protein ligands, and linkers connecting the two, typically large molecular weights (usually 700-1000Da), resulting in poor DMPK characteristics, high clearance rates, and low oral bioavailability, making standalone drug development challenging. Molecular glues have smaller molecular weights and better pharmacokinetic properties, but they often have poor target selectivity, leading to significant toxicity, with clinical doses being very low and indications being limited. Coupling protein degraders into ADCs can effectively “leverage strengths and avoid weaknesses.”
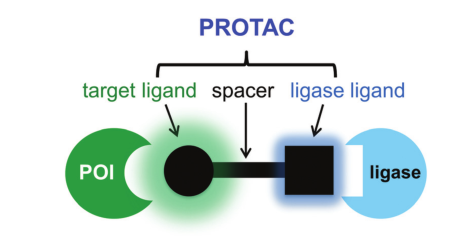
PROTAC Structure Diagram
Orum Therapeutics has developed the TPD2 technology platform, with the first product being an ADC molecule ORM-5029 targeting Her2 using GSPT1 molecular glue as the payload, which has now entered Phase I clinical trials. In vitro activity tests show that ORM-5029 exhibits strong killing activity against various cells, outperforming Enhertu. In in vivo activity tests, it shows efficient and sustained tumor growth inhibition in breast cancer cell models (MDA-MB-453 and HCC1569), with tumor regression observed at a dose of 10mg/kg.
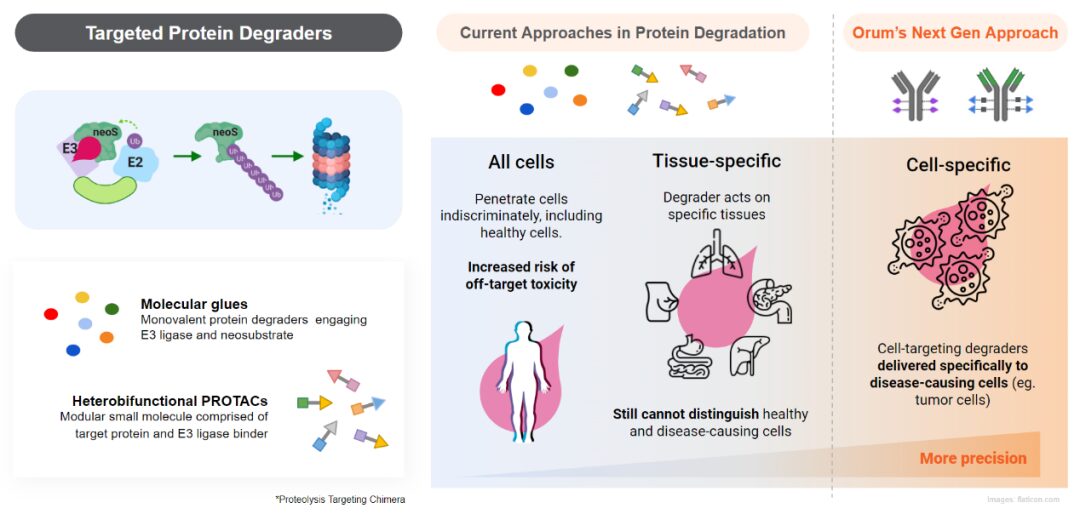
TPD2 Technology (Source: Orum Official Website)
Other payloads also involve nucleic acid compounds, radioactive nuclides, glucocorticoids, antibiotics, and some innovative drugs (e.g., apoptosis inducers, nicotinamide phosphoribosyl transferase inhibitors, protease inhibitors, etc.).
New target payloads for ADCs are still in the early stages and require extensive research to support concept validation. Meanwhile, most new payloads are non-potent cytotoxic drugs, and ensuring that the payload achieves the minimum effective concentration in target tissues is the main challenge.
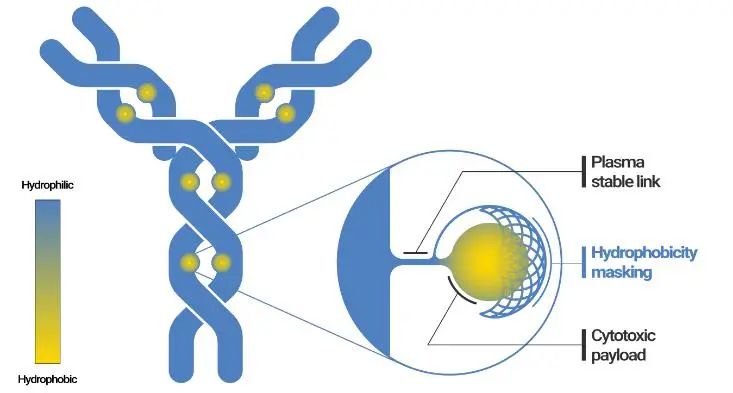
Exploring from the perspective of new linkers.The primary role of existing linkers is to connect the payload with the antibody, releasing the payload in target tissues while further improving their stability in systemic circulation. Additionally, new linkers need to endow more functions, which can be approached from the following three directions:
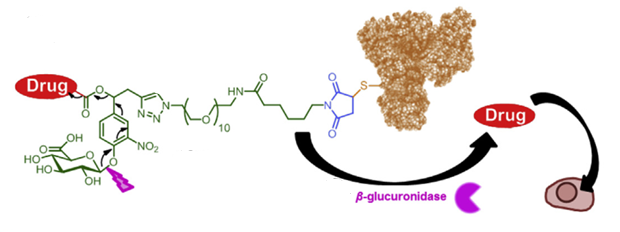
First, develop linkers that can effectively release payloads both intracellularly (in lysosomes) and extracellularly (in the tumor microenvironment), significantly improving the “utilization” of the payload. This will not only address the low permeability issue of ADCs but also expand the range of antibodies that can be used for ADC development. In other words, antibodies that are weakly endocytosed or not endocytosed at all can be used for ADC development, and ADCs can also be developed for targets outside tumor cells.
Reports indicate that Val-Cit-PABC and Val-Ala-PABC linkers can effectively release toxins through both intracellular lysosomal Cathepsin B hydrolysis and “extracellular enzymatic cleavage” to achieve tumor-killing effects. However, due to the instability of the VC linker in mouse plasma, it remains debatable whether its in vivo efficacy comes from tumor microenvironment cleavage or cleavage in blood circulation. Furthermore, due to physicochemical properties, traditional VC linker technology requires further modification for high DAR value ADCs.
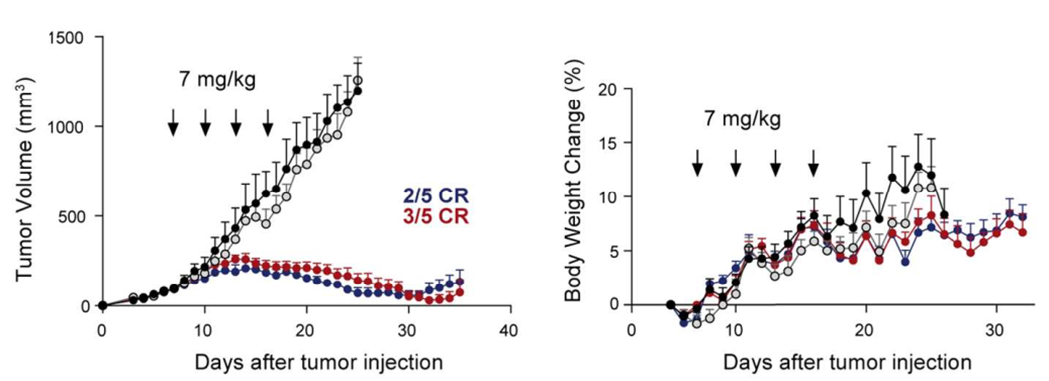
Val-Cit-PABC and Val-Ala-PABC Linkers Can Be Cleaved Extracellularly
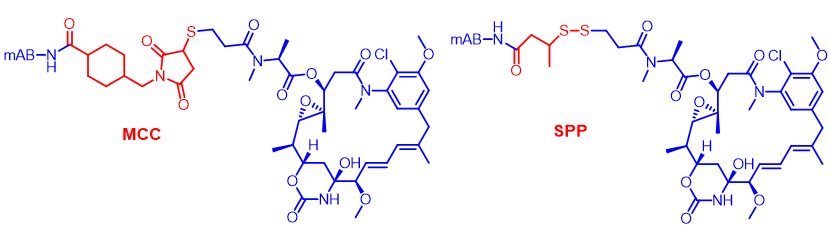
Glutathione can effectively cleave disulfide bonds, with concentrations in tumor tissues being 1000 times higher than in blood, allowing for selective cleavage of disulfide-linked payloads in tumors, releasing toxins and achieving in vivo antitumor effects. The SPP linker possesses this characteristic, demonstrating in vivo efficacy for ADCs composed of both endocytosed and non-endocytosed antibodies.
– Scroll down to view references –
Copyright © 2023 PHARMCUBE. All Rights Reserved.
Welcome to share and cite reasonably, please indicate the source prominently when citing; for reprinting, please leave a message to the WeChat public account or send a message, and indicate the public account name and ID.Disclaimer: The information in this article is for general reference only and should not be used as direct decision-making content. PharmaCube does not assume any responsibility for any losses caused by any entity due to the use of this article’s content.