
Antibody-drug conjugates (ADCs) have become a popular avenue for cancer treatment in recent years. They typically consist of monoclonal antibodies (mAbs) covalently linked to cytotoxic drugs (payloads) through chemically synthesized linkers. ADCs combine the advantages of high specificity targeting and effective killing action, achieving precise and efficient elimination of cancer cells.
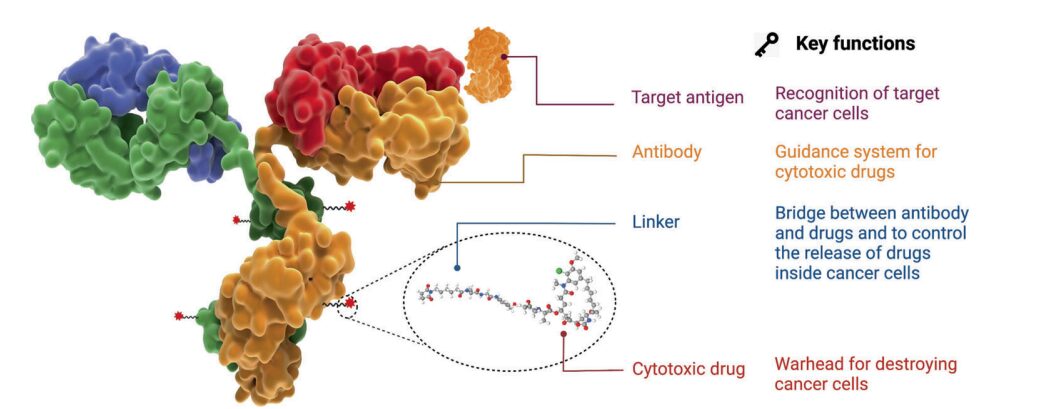
Figure 1. Structure and Characteristics of ADC Drugs
Since the FDA approved the first ADC product, Mylotarg® (gemtuzumab ozogamicin), in 2000, as of December 2021, a total of 14 ADCs have been approved for market use in hematologic malignancies and solid tumors worldwide. Additionally, there are currently over 100 ADC candidates in various stages of clinical trials.
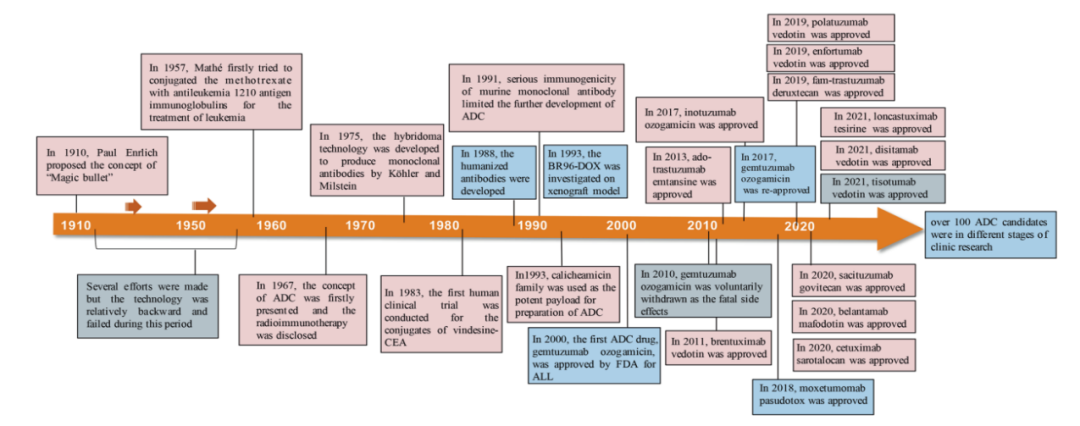
Figure 2. Important Events in the Development and Approval Process of ADC Drugs
However, the development path for innovative oncology products is not always smooth, and the ADC field is no exception. Take the first globally marketed ADC product, Mylotarg®, and the recently failed Phase III clinical trial of Blenrep® as examples.
Blenrep® Phase III Study Failure
-
On November 7, 2022, GlaxoSmithKline’s targeted BCMA ADC product Blenrep® (belantamab mafodotin) failed in a Phase III clinical trial (DREAMM-3) involving patients with a type of blood cancer, as the single-agent treatment did not achieve the primary endpoint of progression-free survival (PFS) compared to the control group receiving pomalidomide combined with low-dose dexamethasone. Previously, Blenrep (belantamab mafodotin) had received accelerated approval from the FDA as a monotherapy for adult patients with relapsed or refractory multiple myeloma (RRMM) who had received at least four prior treatments.
Mylotarg®’s Dual Market Approval
-
In 2000, the FDA accelerated the approval of Pfizer’s targeted CD33 ADC product Mylotarg® as a monotherapy (high dose) for CD33-positive AML adult patients who had experienced their first relapse, were ≥60 years old, and were not suitable for other cytotoxic chemotherapy. This marked the first market entry of Mylotarg®, making it the world’s first commercialized ADC product. However, shortly after its launch, due to safety concerns, Pfizer announced in 2010 that Mylotarg® would be withdrawn from the U.S. market.
-
On September 1, 2017, Pfizer obtained FDA approval again by updating clinical evidence, adjusting drug specifications, and dosing regimens: (1) for newly diagnosed CD33-positive AML adult patients; (2) for children and adults ≥2 years old with CD33-positive, relapsed or refractory AML.
The efficacy and safety of ADCs, therapeutic windows, target limitations, and the heterogeneity of solid tumors and hematologic malignancies all pose challenges to ADC development.
This article aims to explore the key components of ADCs and the mechanisms by which these key factors influence ADC activity by reviewing the development history and mechanisms of ADCs, and to predict the prospects for the development of next-generation ADC products.
Selection of Antigens for ADCs
The target antigens expressed on tumor cells guide ADC drugs in recognizing tumor cells and determine the mechanism of delivering cytotoxic payloads into tumor cells (e.g., endocytosis). Therefore, appropriate selection of target antigens is the primary consideration in ADC development.
-
Firstly, to reduce off-target toxicity, the target antigens should be expressed or predominantly expressed in tumor cells, but expressed little or not at all in normal tissues. Ideally, the antigens should be surface (or extracellular) antigens rather than intracellular antigens, so they can be recognized by circulating ADCs. For example, in certain types of tumors, the expression of the HER2 receptor is approximately 100 times higher compared to normal cells, which lays a solid foundation for the development of ado-trastuzumab emtansine (TDM-1, KADCYLA®), fam-trastuzumab deruxtecan (DS-8201a, Enhertu®), and disitamab vedotin (RC48, Aidixi®);
-
Secondly, the target antigens should be non-secreted, as circulating secreted antigens can lead to unwanted binding of ADCs outside the tumor site, reducing tumor targeting and increasing side effects;
-
Thirdly, the target antigens should ideally be internalized upon binding with the corresponding antibody, allowing the ADC-antigen complex to effectively enter cancer cells, followed by appropriate intracellular transport pathways and rapid release of the cytotoxic payload.
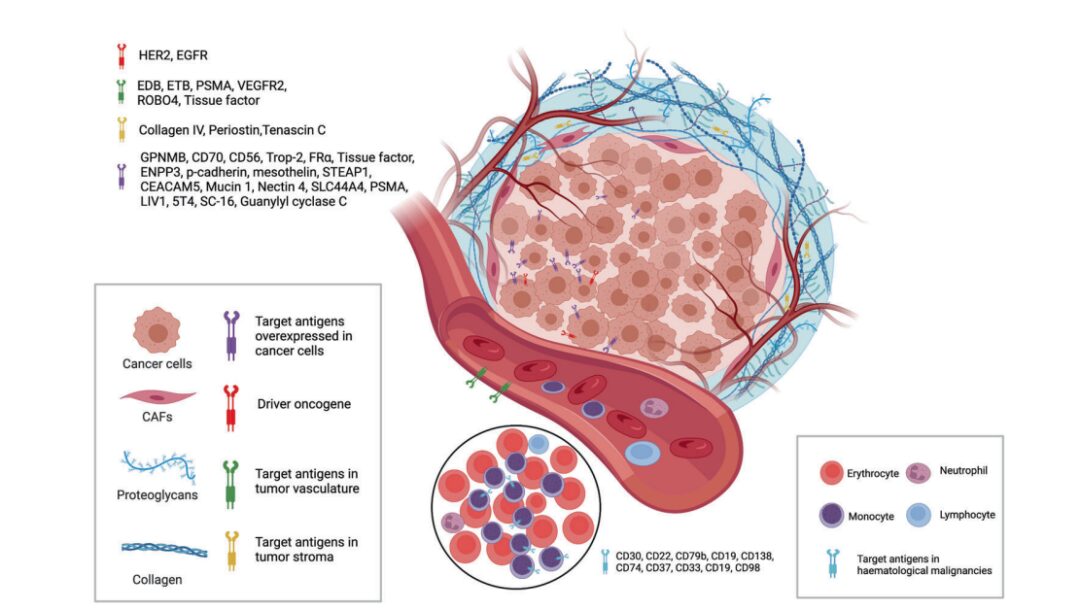
Figure 3. Available Targets from Tumor Cells and Tumor Microenvironment for ADCs
Currently, as shown in the figure above, the approved ADC drugs typically target specific proteins that are overexpressed in cancer cells, including HER2, trop2, neectin4, and EGFR in solid tumors, as well as CD19, CD22, CD33, CD30, BCMA, and CD79b in hematologic malignancies. Driven by basic research in oncology and immunology, the selection of ADC target antigens is gradually expanding from traditional tumor cell antigens to targets in the tumor microenvironment, such as stroma and vascular systems. New evidence emerging from preclinical and clinical studies indicates that components of the neovascular system, subendothelial extracellular matrix, and tumor stroma may be valuable target antigens for ADC drug development.
Selection of Antibodies for ADCs
Targeting antibodies for tumors is crucial for the specific binding between the target antigen and ADC. In addition to having high binding affinity for the target antigen, the ideal antibody should facilitate effective internalization and exhibit low immunogenicity and a long plasma half-life.
In the early stages of ADC drug development, murine antibodies were primarily used, but they had high failure rates due to severe immunogenic side effects. With the advent of recombinant technology, murine antibodies have mostly been replaced by chimeric antibodies and humanized antibodies. Currently, ADCs are increasingly using fully humanized antibodies with significantly reduced immunogenicity. Most antibodies used in ADC drugs are immunoglobulin G (IgG) antibodies, including four subclasses: IgG1, IgG2, IgG3, and IgG4.
-
IgG1 is the commonly used antibody subclass for ADCs, as IgG1 is the most abundant in serum and can induce potent effector functions through high binding affinity to Fc receptors, such as antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC). These Fc-mediated effector functions play a crucial role in the anti-cancer activity of antibody drugs.
-
Due to rapid clearance, IgG3 is rarely used for ADCs. Unlike the other three subclasses with a half-life of about 21 days, IgG3 has a half-life of only about 7 days in serum.
-
IgG2 often exhibits a tendency to form dimers and aggregates in vivo, leading to decreased concentrations of ADC drugs.
-
IgG4 can induce ADCP; however, IgG4 is an anomalous dynamic antibody with Fab arm exchange, leading to reduced potency and ineffective targeting.
Regarding the internalization of the antibody-antigen complex, the efficiency mainly depends on the binding affinity between the antibody and tumor cell surface antigens, where higher affinity usually leads to faster internalization. However, antibodies with high antigen affinity may conversely reduce penetration into solid tumors. Treating solid tumors is more complex than treating hematologic malignancies because of the presence of a “binding site barrier (BSB)” in solid tumors, where the extremely strong binding between antibodies and antigens leads to ADC being trapped near the blood vessels after extravasation, with less penetration into tumor cells far from the vessels. Therefore, an optimal balance of reasonable affinity between antigens and antibodies should be achieved to balance rapid uptake in target cells and anti-cancer efficacy.
In addition to binding affinity, another factor affecting tumor penetration is the size of the antibody. The large molecular weight of IgG antibodies (about 150kDa) often limits their ability to permeate through capillaries and stroma in tumor tissues. Therefore, early ADCs primarily targeted hematologic malignancies. To better apply ADCs in solid tumor treatments, researchers have attempted to miniaturize antibodies by removing the Fc fragment. Miniaturized antibodies not only maintain high affinity and specificity but also penetrate solid tumors more easily through blood vessels, significantly enhancing their killing effects on solid tumors. However, such changes may lead to a shortened half-life in vivo. Therefore, when designing ADCs with miniaturized antibodies, various factors should be comprehensively considered for reasonable optimization.
Selection of Linkers for ADCs
The linker in ADCs connects the antibody to the cytotoxic drug. It is one of the key factors associated with ADC stability and the release curve of the payload, thus being crucial for the final therapeutic indicators of ADCs.
An ideal linker should not induce ADC aggregation, is expected to limit the premature release of the payload in plasma, and facilitate the release of active drugs at the desired targeting sites. Based on the metabolic pathways in cells, most ADC drugs use two types of linkers: cleavable linkers and non-cleavable linkers. Cleavable linkers utilize the environmental differences between systemic circulation and tumor cells to accurately release free cytotoxic drugs, which can be further divided into chemical cleavable linkers (hydrazone bonds and disulfide bonds) and enzyme-cleavable linkers (glucuronide bonds and peptide bonds). Hydrazone is a typical acid-sensitive (pH-sensitive) linker.
Cleavable linkers utilize the environmental differences between systemic circulation and tumor cells to accurately release free cytotoxic drugs, which can be further divided into chemical cleavable linkers (hydrazone bonds and disulfide bonds) and enzyme-cleavable linkers (glucuronide bonds and peptide bonds). Another chemically sensitive cleavable linker is sensitive to reductive glutathione (GSH). GSH plays a crucial role in maintaining the intracellular redox balance during cell survival, proliferation, and differentiation. The concentration of GSH in the blood is much lower than the intracellular concentration in cancer cells. Therefore, this type of linker can remain stable in the blood system while specifically releasing active payloads in cancer cells.
Peptide-based linkers are sensitive to lysosomal proteases and have been used in many ADCs. Lysosomal proteases, such as cathepsin B, are often overexpressed in cancer cells, allowing for accurate drug release near tumors. Additionally, due to the presence of protease inhibitors in the blood, enzyme-cleavable linkers are usually stable in systemic circulation, thus reducing the various risks associated with premature cleavage. Among the approved ADC drugs, 9 out of 14 use peptide-based linkers.
Non-cleavable linkers (e.g., thioether or maleimide-based) are inert to the common chemical and enzymatic environments in vivo. Due to the improved plasma stability, the main advantage of non-cleavable linkers is their lower off-target toxicity, but the bystander effect of the payload is affected.
Selection of Payloads for ADCs
Cytotoxic payloads are the warheads that exert cytotoxicity once ADCs are internalized by cancer cells. Since only about 2% of ADCs reach the targeted tumor site after intravenous administration, high-potency payloads (IC 50 in the nM and pM range) are key to ADC development. Additionally, these compounds should remain stable under physiological conditions and possess available functional groups for binding to antibodies.
Currently, the cytotoxic payloads used in ADCs mainly include potent microtubule inhibitors, DNA damaging agents, and immunomodulators (Table 1).
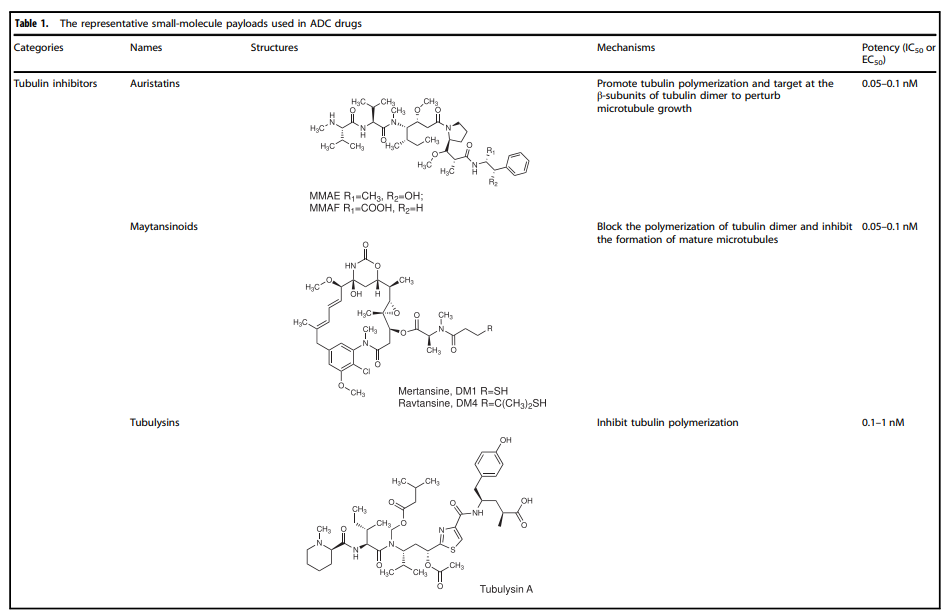
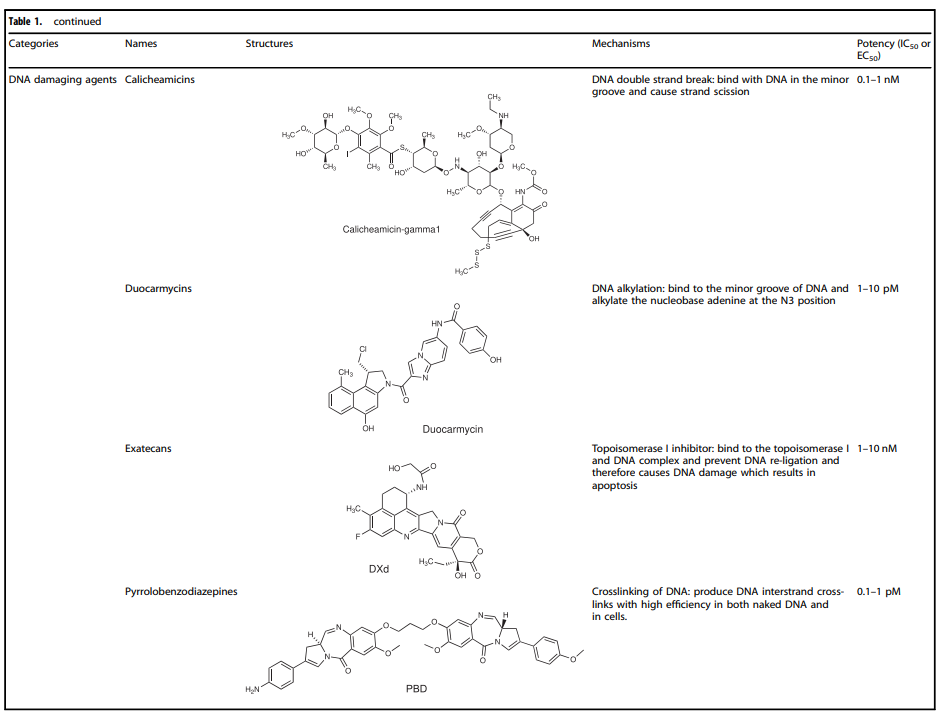
Table 1. Representative Small Molecule Payloads Used in ADC Drugs
Microtubule Disruptors
Microtubules are a major component of the cytoskeleton and play an important role in cell division, especially in the rapid proliferation of tumor cells. Microtubule protein inhibitors have become important anti-cancer drugs. Auristatins are significant payloads used in ADCs, with monomethyl auristatin E (MMAE) or monomethyl auristatin F (MMAF) becoming mainstream payloads in ADC development. Among the 14 approved ADC drugs, 5 use MMAE/MMAF as payloads.
Maytansinoid Derivatives (DM1 and DM4)
Maytansine is a highly effective microtubule assembly inhibitor that can induce mitotic arrest in cells. However, this structure is challenging to conjugate due to the lack of reactive functional groups. To overcome this problem, a series of highly effective derivatives containing SMe groups were created. The first examples of this class of molecules are DM1 and DM4, which contain methylthioacetyl instead of the natural N-acetyl.
The FDA approved ado-trastuzumab emtansine (TDM-1, KADCYLA®) in 2013 as the first ADC drug conjugated with maytansinoid derivatives. Additionally, microtubule inhibitors (microtubule inhibitors A-D, isolated from slime molds) represent another class of microtubule polymerization inhibitors that exhibit good anti-cancer activity. For example, EC1169, a microtubule lysosome B hydrazone conjugate targeting prostate-specific membrane antigen (PSMA), is currently undergoing clinical trials (NCT02202447).
DNA Damaging Agents
Compared to the IC50 in the nmol range of microtubule inhibitors (half the maximum inhibitory concentration), the IC50 values of DNA damaging agents can reach the pmol level, making ADCs conjugated with DNA damaging agents sometimes more effective and potentially able to work independently of the cell cycle (compared to microtubule inhibitors, which primarily work during mitosis), and they can even be used in cells with low antigen expression.
The detailed mechanisms involved in DNA damaging agents primarily include: (i) DNA double-strand breaks, such as calicheamicin; (ii) DNA alkylation, such as duocarmycin; (iii) DNA intercalation, such as topoisomerase I inhibitors; (iv) DNA cross-linking, such as pyrrolobenzodiazepine (PBD).
It is the various functions of the payload itself that inhibit tumor cell growth or directly kill tumor cells that enrich the mechanism of action of ADC drugs.
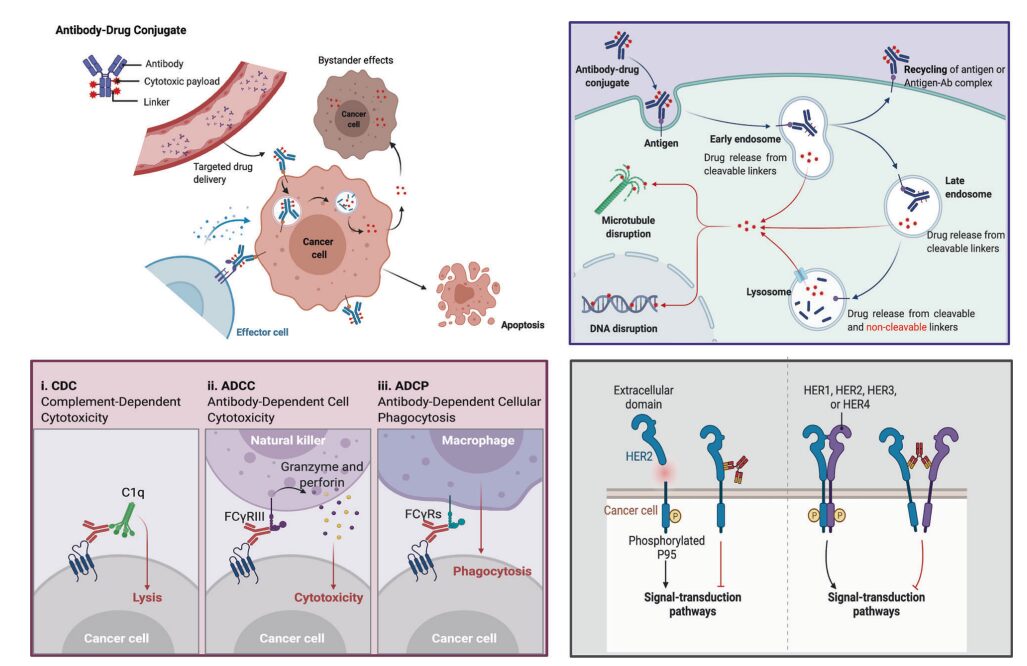
Figure 4. Overview of Mechanisms by Which ADCs Kill Cancer Cells
Upper right: Main core mechanisms of ADCs; lower left: The antibody component of ADCs interacts with immune effector cells, triggering anti-tumor immunity, including CDC, ADCC, and ADCP effects; lower right: The antibody component of ADCs retains its active characteristics, thus interfering with target functions and inhibiting downstream signaling pathways that suppress tumor growth.
Despite the numerous anti-tumor mechanisms stemming from the inherent tumor-targeting elimination effect of ADCs, the cytotoxic effects received by antibodies, and the various anti-tumor mechanisms of the payload, the key to ADC success lies in the conjugation method of the antibody, payload, and linker.
Conjugation Methods
The conjugation site and method determine the drug-to-antibody ratio (DAR) of the ADC, and the conjugation site has a significant impact on the stability of the ADC and its pharmacokinetic – pharmacodynamic characteristics. High drug loads often lead to rapid plasma clearance, while low DAR ADCs exhibit weaker activity.
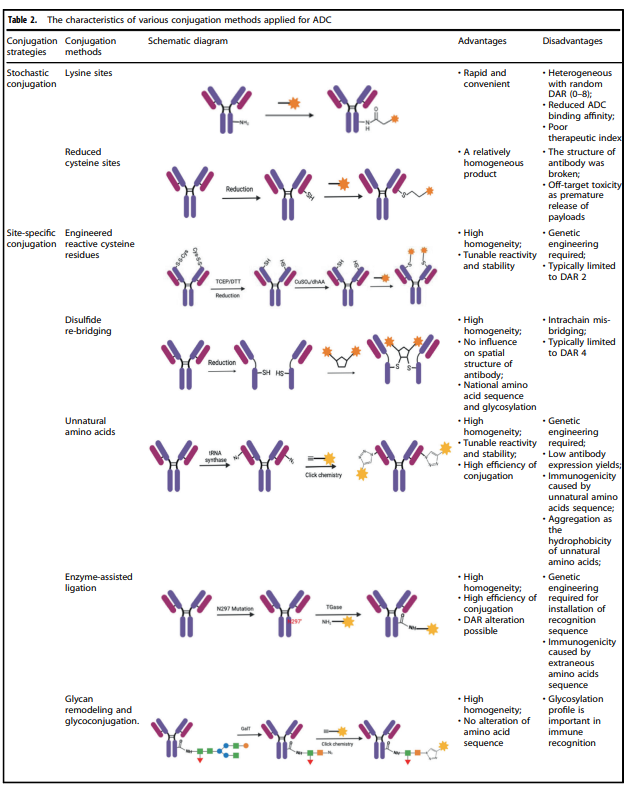
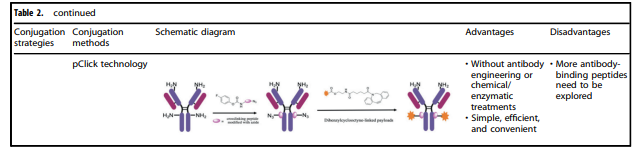
Table 2. Characteristics of Various Conjugation Methods Applied to ADCs
Table 2 summarizes the characteristics of various conjugation methods commonly used in ADCs. Among these, chemical coupling and enzymatic coupling are the two most frequently used methods for linking antibodies and payload components.
Chemical Coupling
The accessible amino acid residues on the surface of antibodies are coupled in chemical coupling by linking the handle portion of the linker to the amino acid residue portion of the antibody, avoiding the complexity of identifying suitable mutation sites and the potential challenges of scaling up and optimizing cell culture. Early chemical coupling methods had great randomness in DAR and conjugation sites, resulting in significant differences in the produced ADCs, leading to uneven quality.
Enzymatic Coupling
By using genetically encoded amino acid tags inserted into the antibody sequence, payload attachment can be achieved in a highly selective manner. These tags are specifically chosen to be recognized by enzymes capable of executing site-specific coupling, such as formylglycine-generating enzyme (FGE), microbial transglutaminase (MTG), sortase, or tyrosinase.
Potential Development Directions for ADCs
With the continuous optimization of conjugation technologies and advancements in antibody engineering techniques, more ADC products targeting other antigens are under clinical exploration. Examples include Trop2 ADC, Claudin18.2 ADC; among them, PD-L1 ADC is also being explored by several companies.
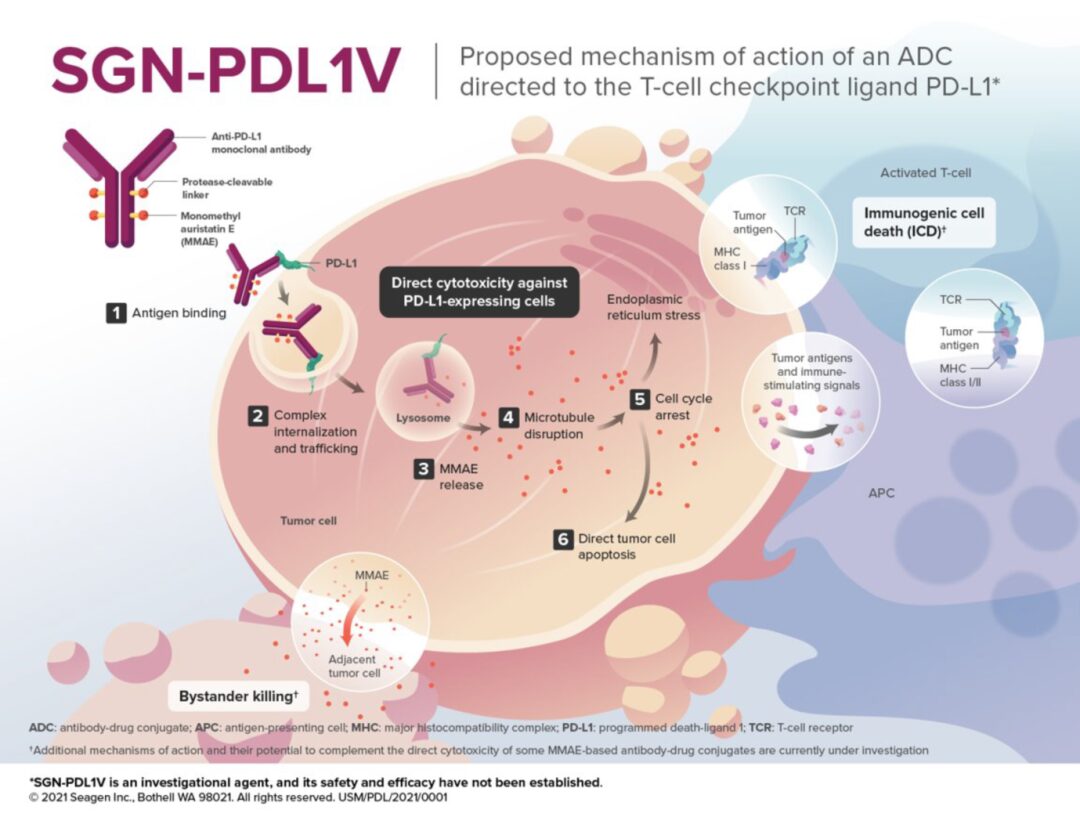
Figure 5. Mechanism of Action of SGN-PDL1V (Source:2021 SITC)
For example, SGN-PDL1V, a novel ADC product targeting PD-L1 in the early development stages by Seagen, uses MMAE as the payload. MMAE (monomethyl auristatin E) is the most widely used payload in ADCs, accounting for about 30% of the total, primarily exerting effective mitotic inhibition by inhibiting microtubule polymerization.
ADCs show drug concentrations >100 times in tumor tissues, significantly expanding the therapeutic window of cytotoxic agents and effectively reducing the side effects caused by systemic chemotherapy, thus facilitating the exploration of combined use of ADCs with other cancer treatment modalities.
Furthermore, the linker of SGN-PDL1V is protease-cleavable. The antibody is a fully human anti-PD-L1 monoclonal antibody (Seagen PD-L1 monoclonal antibody). Seagen’s PD-L1 monoclonal antibody utilizes a human IgG1-Fc backbone, engineered to eliminate the effector functions of the Fc segment, including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP).
Additionally, given that single-target antibodies often exhibit high resistance rates, dual-target bispecific antibodies as ADCs are also being explored by some companies.
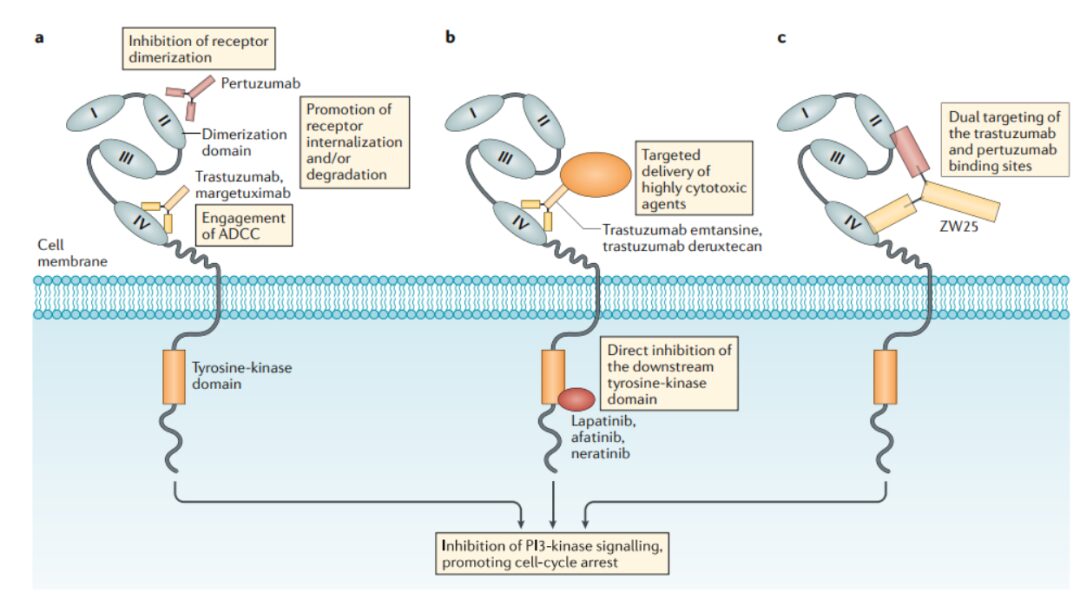
Figure 6. Mechanism of Action of HER2-Targeting Drugs
This field is currently in the early exploration stage, with the fastest progress being ZW-49, a bispecific antibody ADC developed by Zymeworks utilizing its proprietary Azymetric™ Bispecifics and ZymeLink™ ADC platforms. It can specifically bind to two non-overlapping epitopes of the HER2 receptor, namely the binding sites of pertuzumab and trastuzumab. It aims to achieve good therapeutic effects in patients resistant to trastuzumab, pertuzumab, and even new ADC drugs like TDM-1 in clinical applications.
We look forward to more novel ADCs being commercialized in the future to benefit a wider range of patients in the fields of solid tumors and hematologic malignancies.
References:
1. Signal Transduction and Targeted Therapy (2022) 7:93
2. Bioorg Chem. 2021 Nov;116:105366.
3. Oh DY, et al. Nat Rev Clin Oncol. 2019;10.1038/s41571-019-0268-3.
4. Mark D. Pegram, et al. Mol Cancer Ther; 20(8) August 2021.
