Introduction
As one of the most active innovative drug development areas at present, Antibody-Drug Conjugates (ADCs) are rising at an astonishing pace. The global industry interest continues to heat up, with ADC innovative drugs being approved one after another. As of March 2025, a total of 17 ADC products have been approved for market release worldwide. Collaborations and licensing transactions in the ADC field are steadily advancing, with at least 8 transactions occurring at the beginning of 2025, totaling a potential transaction value of over $10 billion. Meanwhile, an increasing number of ADC drugs in clinical stages are demonstrating significant therapeutic potential.
Structure of Antibody-Drug Conjugates (ADCs)
Antibody-Drug Conjugates (ADCs) are a type of innovative antibody drug formed by the conjugation of antibodies or antibody fragments targeting specific antigens with an effective payload through a linker (as shown in Figure 1). Compared to traditional antibody drugs, ADC products possess both macromolecular and small molecular properties, combining the potent effects of traditional small molecule drugs with the targeting capabilities of antibody drugs.
ADCs deliver small molecule cytotoxins precisely to tumor cells, tumor microenvironments, or other target cells through a “biological missile” mechanism. Upon reaching their destination, they are internalized and release cytotoxic drugs, thereby reducing systemic toxicity and exerting a more selective anti-tumor effect.
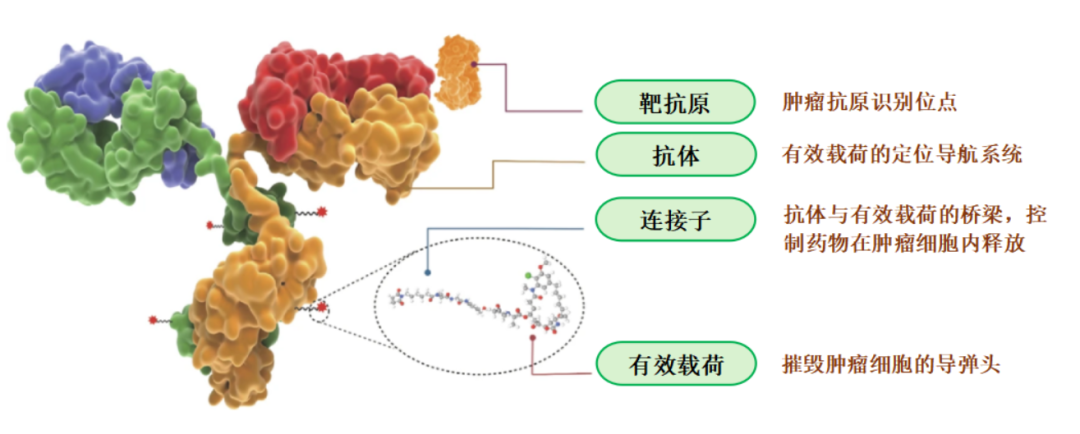
Figure 1 Structure of ADC Drugs
Globally Approved ADC Drugs
As one of the most promising strategies for precise tumor treatment today, ADCs cleverly assemble antibodies, linkers, and effective payloads into a unique drug structure, achieving precise targeting and effective killing of tumor tissues.
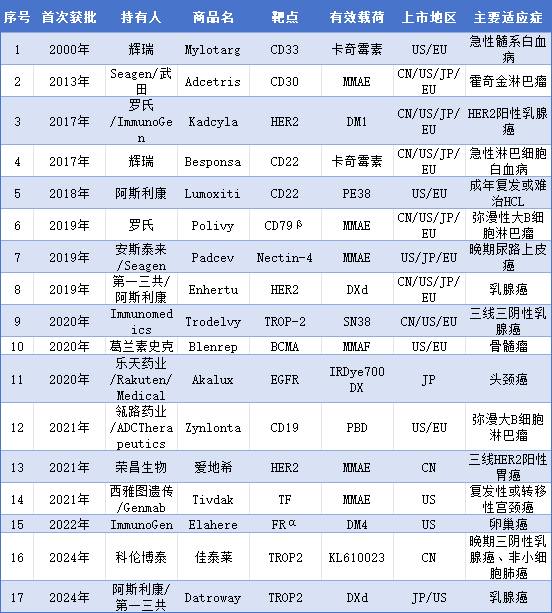
As of February 2025, a total of 17 ADC products have been approved for market release worldwide (see Table 1 below). The targets involved in these approved products cover popular targets such as HER2, TROP2, EGFR, FRα, TF, as well as mainstream toxin molecules like MMAE, DXd, SN38, and DM4.
Table 1 Summary of Globally Approved ADC Drugs
Summary of ADC Drugs Under Research in China
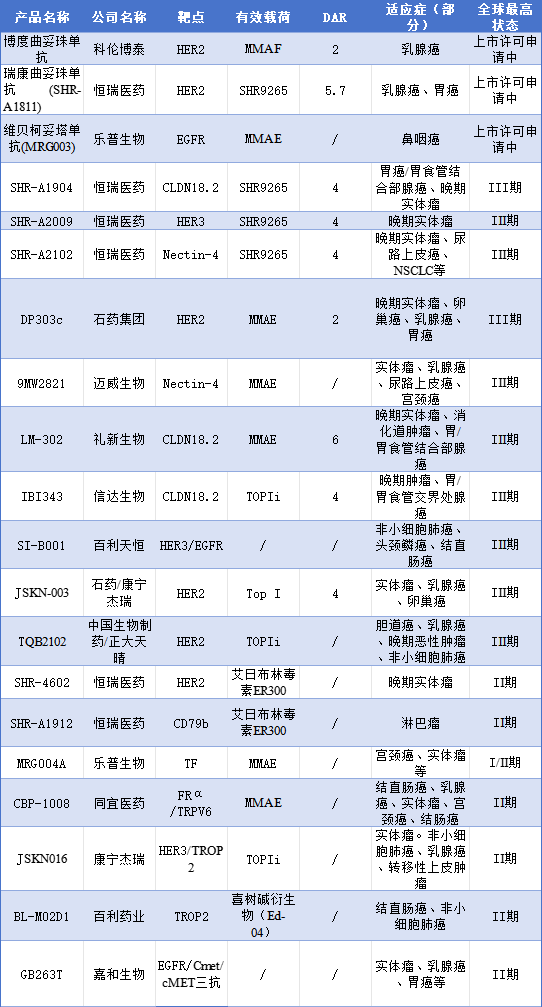
ADCs are entering a period of explosive growth, with a surge of products emerging and intense competition in new drug development. Data shows that at least a thousand ADC clinical trials are currently underway. The following summarizes some of the ADC drugs under research as of February 2025 (Table 2).
Table 2 Summary of ADC Drugs Under Research in China as of 2025 (Excerpt)
Commercial Landscape of ADCs
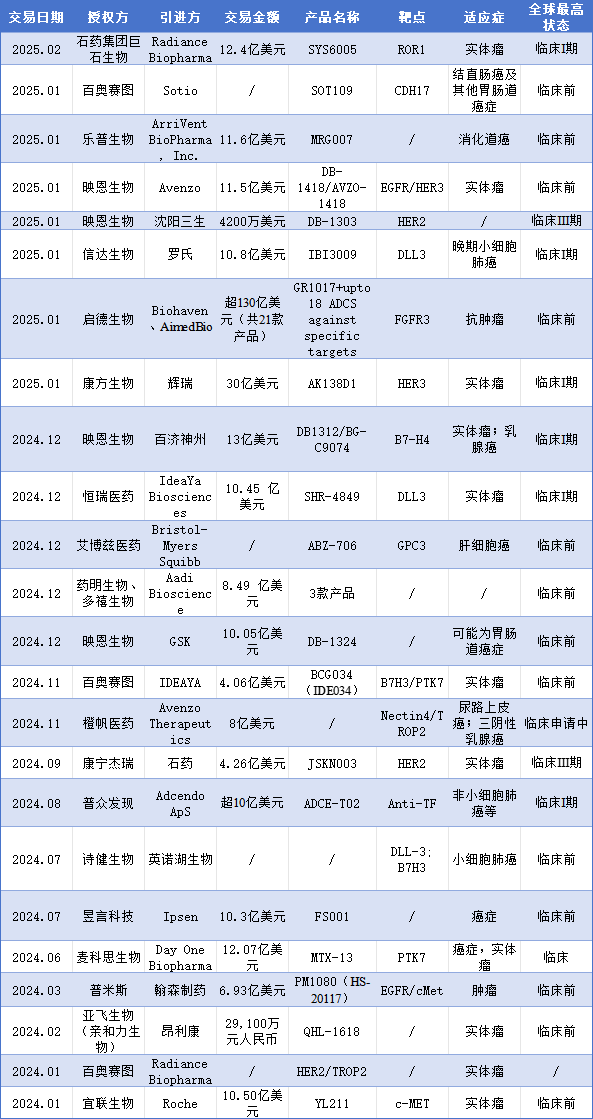
In the forefront of pharmaceutical innovation, the global ADC drug market is in a rapid development phase. Today, China has become a source of ADC innovation, with impressive external licensing transactions. According to incomplete statistics, at least 16 ADC products reached transactions from 2024. Entering 2025, the trend of ADC drug transactions continues. As of February 2025, based on disclosed data, the ADC field has welcomed at least 8 transactions, with a total potential transaction value exceeding $10 billion.
Table 3 Summary of Some ADC Commercial Transactions (January 2024 – February 2025)
Non-Clinical Requirements for ADC Products*
The structure and functional characteristics of ADCs are complex. Their non-clinical research should follow the general principles of innovative drug development while considering various factors such as the characteristics of the antibody, linker, and payload in the drug, indications, administration routes, and dosing regimens, employing a specific problem analysis evaluation strategy.
1. Pharmacological Studies
-
In the early development of ADCs, pharmacological/pharmacodynamic studies should be conducted both in vitro and in vivo.
√ Conduct pharmacological/pharmacodynamic studies on the overall ADC
√ Study the pharmacological effects of each component of the ADC, mainly including target antigen binding specificity, target antigen binding activity, possible pharmacological effects related to target antigens, Fc effects, such as FcRn, FcγR receptor family, C1q binding activity, antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), etc.
√ If the payload is a new compound, relevant non-clinical studies should be conducted according to the requirements for new compounds.
√ Mechanistic studies on the effective payload or major pharmacologically active metabolites should be conducted, or relevant literature data should be provided.
2. Safety Pharmacology Studies
-
Similar to safety pharmacology studies in innovative drug development, the impact of ADCs on important system functions (including cardiovascular, respiratory, and central nervous systems) should be assessed before clinical trials. This can be conducted separately or combined with general toxicology studies.
-
Special Considerations: If the payload is a new compound, a separate hERG test should be conducted to assess the risk of QT interval prolongation.
3. Pharmacokinetics (PK) Studies
1. Selection of Detection Methods
-
Analytical methods for conjugated antibodies, total antibodies (conjugated antibodies and naked antibodies), and effective payloads should be established, and corresponding method validation should be completed.
-
The systemic exposure of the effective payload may be low, and radioactive labeling methods are usually used to track its ADME in vivo.
2. PK studies of ADC drugs typically include: in vitro stability, blood concentration-time curves, absorption, distribution, metabolism, excretion (ADME), plasma protein binding, and effects on drug-metabolizing enzyme activity and transporters.
3. Special Considerations
-
If the effective payload or the main free molecule after ADC in vivo cleavage is a new compound, studies on its plasma protein binding, tissue distribution, metabolism, excretion/substance balance, and effects on drug-metabolizing enzymes and transporters should be conducted.
-
For entirely new linkers, attention should be paid to their metabolic status in vivo during development.
-
Generally, tissue distribution studies for ADCs are not required, but for ADCs intended to target specific tissue lesions for therapeutic effects, tissue distribution studies should be conducted in relevant animal species to clarify their targeting tissue distribution characteristics.
4. Toxicology Studies
1. General Toxicology
-
Similar to general toxicology studies for conventional innovative drugs, ADC drugs should conduct single/repeated dose toxicity studies in relevant animal species.
-
ADCs differ from general antibody drugs, as their toxic reactions mainly stem from the effective payload, and toxicity assessments of the effective payload can generally be achieved through overall ADC administration.
-
For entirely new or poorly characterized toxic effective payloads, general toxicity study principles for innovative small molecule compounds should be followed.
-
Generally, separate toxicity evaluations for linkers and naked antibodies are not required.
2. Genotoxicity Studies
-
ADC and its antibody components usually do not require genotoxicity testing.
-
If the effective payload is a new compound, genotoxicity testing should be conducted.
-
If sufficient data indicate that the target analyte has genotoxicity, genotoxicity studies are not required.
3. Toxicokinetics
-
Toxicokinetic studies of ADCs should be conducted alongside their toxicology studies.
4. Formulation Safety
-
If administered via injection, local irritation and hemolysis should be assessed.
5. Other Toxicity Assessments
-
Studies on reproductive toxicity, carcinogenicity, immunogenicity/immunotoxicity, phototoxicity, tissue cross-reactivity, etc., should be conducted as appropriate based on the action characteristics and indications of the ADC product.
Conclusion
Overall, the ADC field is in a golden period of rapid development, with technological innovation, market expansion, and diversification of indications continuing to drive it as an important pillar of future targeted therapies. The year 2024 is undoubtedly a fruitful year, and I believe that 2025 will be an even more remarkable year for ADC products.
*Reference: CDE “Technical Guidelines for Non-Clinical Research of Antibody-Drug Conjugates (No. 46, 2023)”
Submitted by: Colin Li Kang Pharmaceutical Affairs Department
Suzhou Colin Li Kang Pharmaceutical Technology Co., Ltd.

Quality | Integrity | Efficiency | Benevolence