From the beginning of their development, ADC drugs have faced the problem of a very small therapeutic index, leading to a narrow therapeutic window. To address this issue, many attempts have been made, and three generations of ADC technology have now been developed.

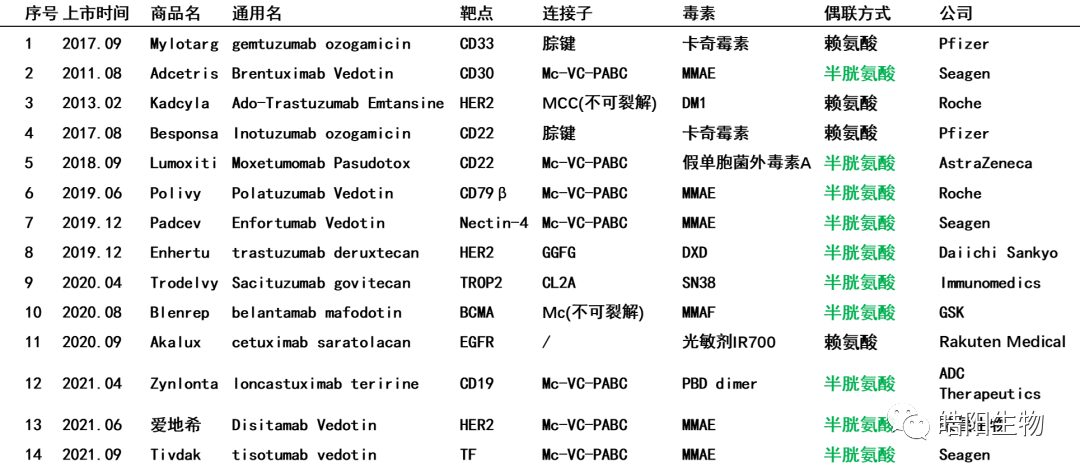
Currently, 71% of the marketed ADCs use inter-chain disulfide bond coupling, as shown in the table below. This highlights the importance of disulfide bonds. Today, I will share with you the structure of antibody disulfide bonds, ADCs based on partially reduced coupling of disulfide bonds, ADCs based on fully opened coupling of disulfide bonds, ADCs based on bridged coupling of disulfide bonds, and the consistency issue of ADC’s DAR value.
1. Structure of Antibody Disulfide Bonds
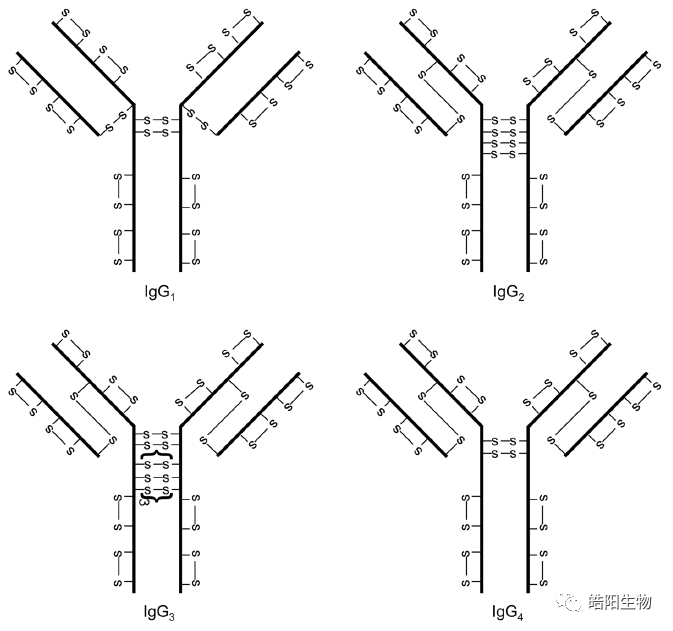
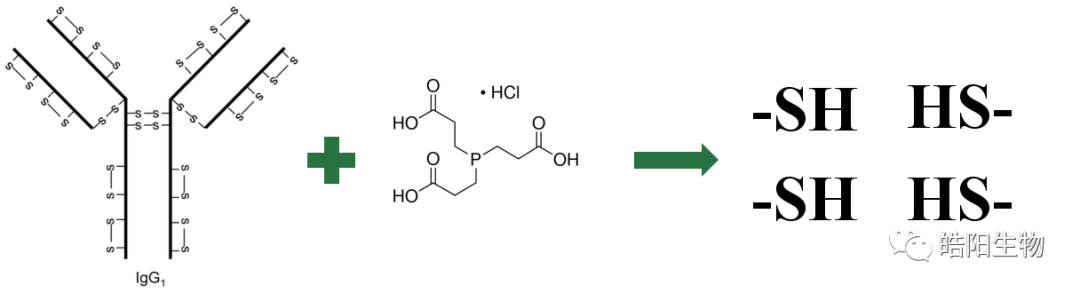
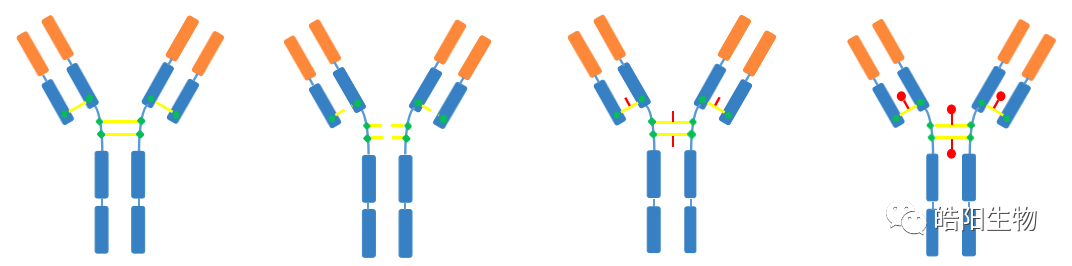
The structure of disulfide bonds in four different types of antibodies is shown in the figure below. Among them, IgG1 is the most widely used in ADCs, and its structure consists of two heavy chains and two light chains, linked by four inter-chain disulfide bonds. There are also intra-chain disulfide bonds on the light and heavy chains, but they are harder to reduce, so the inter-chain disulfide bonds are preferentially reduced, and then conjugated to the small molecule toxin through a maleimide and thiol addition reaction.
During the assembly process of the antibody, mismatches may occur in the disulfide bonds. For example, in the figure below, the purity of the naked antibody is 91.9%. During the ADC process, a reducing agent is required to open its disulfide bonds, and the final purity of the ADC reaches 94.9%. The possible reason is that the inter-molecular disulfide bonds are opened by the reducing agent, leading to increased purity.

2. ADCs Based on Partially Reduced Coupling of Disulfide Bonds
The figure below shows ADCs based on partially reduced disulfide bond coupling, with DAR values of 3.5, 3.8, and 4.0 respectively. When all four disulfide bonds of IgG1 are fully opened, there are eight reactive sites, which requires changing the reduction reaction conditions to open some disulfide bonds. Typically, the amount of reducing agent is adjusted to control the amount of reduced thiols, thereby regulating the final DAR value.

Tri(2-carboxyethyl)phosphine is used for the reduction of antibody disulfide bonds due to its mild reaction conditions, good water solubility, and lack of odor. The figure below illustrates the reduction reaction.
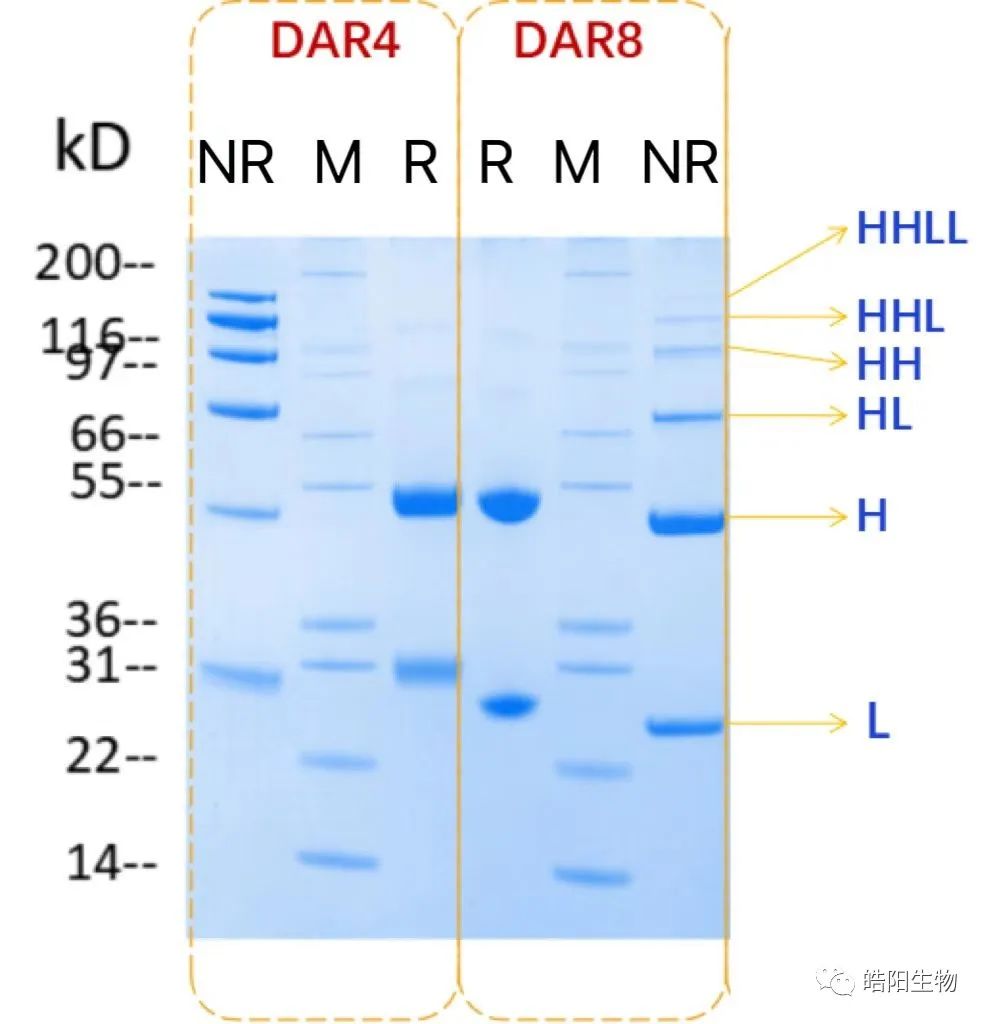
During the preparation of ADCs, the degree of reduction of inter-chain disulfide bonds can be observed through SDS-PAGE, as shown in the figure below. M represents the standard protein, R represents the reduced sample, NR is the non-reduced sample, H is the heavy chain, and L is the light chain. Since the conjugation occurs through the thiols opened by the reduced disulfide bonds, different bands appear in the gel image. This can be illustrated by comparing the gel images of ADCs with DAR values of 4 and 8. It can be seen that the ADC with DAR4 has its four inter-chain disulfide bonds partially opened, while DAR8 requires complete opening, resulting in different band proportions.
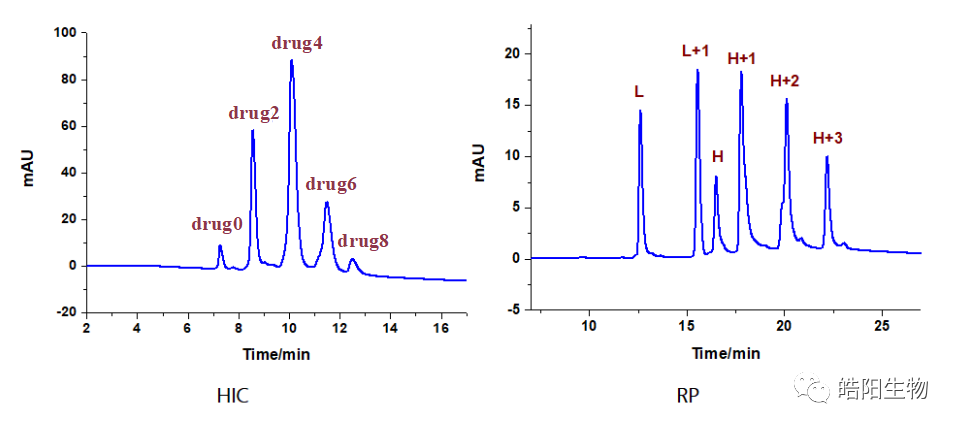
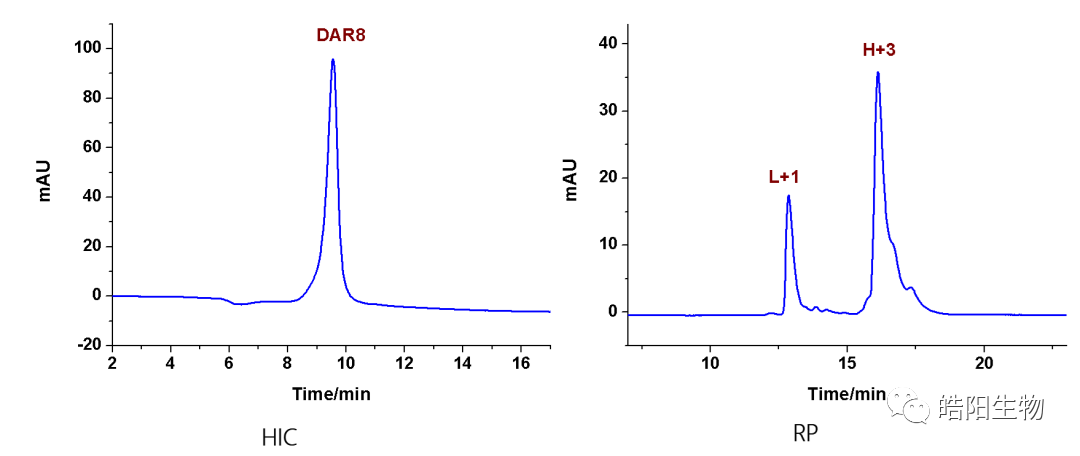
Hydrophobic interaction chromatography is usually used to analyze DAR values. Drug0-Drug8 represent antibodies linked with 0 to 8 small molecule drugs. The figure below shows the hydrophobic interaction chromatography of ADC samples prepared by conjugating Herceptin with MMAE, with a DAR value of 4.04. For samples that cannot be analyzed by hydrophobic interaction chromatography, such as when the chromatographic peaks of Drug0-Drug8 are difficult to distinguish, reversed-phase liquid chromatography can be used for judgment, where L represents the light chain, and H represents the heavy chain, +1, +2, and +3 represent one to three small molecule drugs linked.

3. ADCs Based on Fully Opened Coupling of Disulfide Bonds
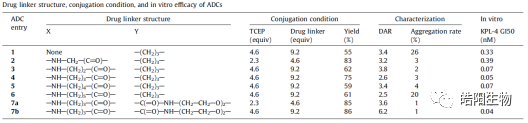
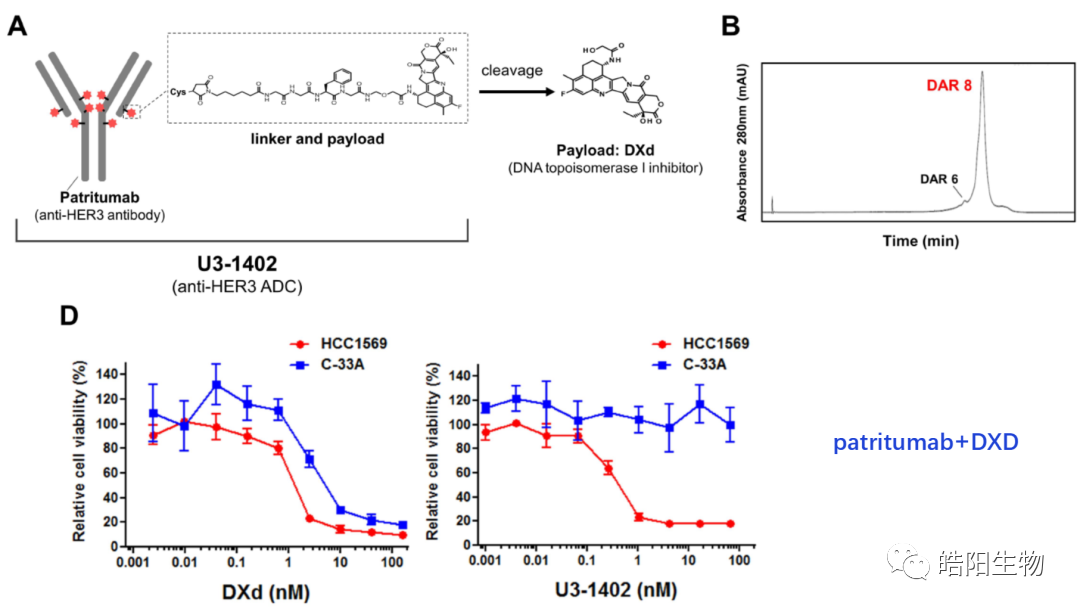
By fully reducing the four inter-chain disulfide bonds of IgG1, eight thiols are obtained, which can theoretically bind with eight small molecules bearing maleimide functional groups, resulting in ADCs with a DAR value of 8. For example, the figures and tables below show the conjugation of Exatecan derivatives to antibodies through different linkers. The changes in functional groups will affect the purity and DAR value of the ADC; the best structure, such as deruxtecan, shows a single DAR8 peak in the hydrophobic chromatography, while the reversed-phase chromatography shows one and three small molecules attached to the light and heavy chains, respectively. Similarly, based on this small molecule, antibodies targeting different targets can be replaced to obtain U3-1402.

4. ADCs Based on Bridged Coupling of Disulfide Bonds
For ADCs with a DAR of 4, there are other products, such as drug2, drug6, drug8, etc. After the antibody is reduced, the four opened disulfide bonds can be reconnected using bifunctional reagents that can react with thiols, but a third functional group is introduced in the linker. This functional group can covalently bind to the functional groups on the toxin, and this method can increase the content of DAR4 by more than 30%.
The figure below uses divinylpyrimidine as a bridging reagent to couple with MMAE, showing a significant increase in the DAR4 component. However, the drawback of this method is that two thiols on the same heavy chain may bridge together, as shown in the gel image. Additionally, this reaction requires copper ion catalysis, and the amount of toxin used is very large, reaching 40 times greater than that required for conjugation using maleimide functional groups.

The scheme below uses bisulfide as a bridging reagent for DAR4 ADCs, where the DAR4 component accounts for 78%, and the ADC prepared by this process has good stability in blood.

5. Consistency Issues and Development Trends of ADC’s DAR Value
The development trend of DAR value regulation can be summarized in the figure below, starting from over 70 amino acid couplings of 1-8 small molecules, such as the DAR value of Trastuzumab emtansine (T-DM1), evolving to 4 disulfide bonds coupling with 1-8 small molecules, and now 2 sugar chains coupling with 2-4 small molecules. As the coupling sites become smaller, the complexity of the coupling reaction increases.

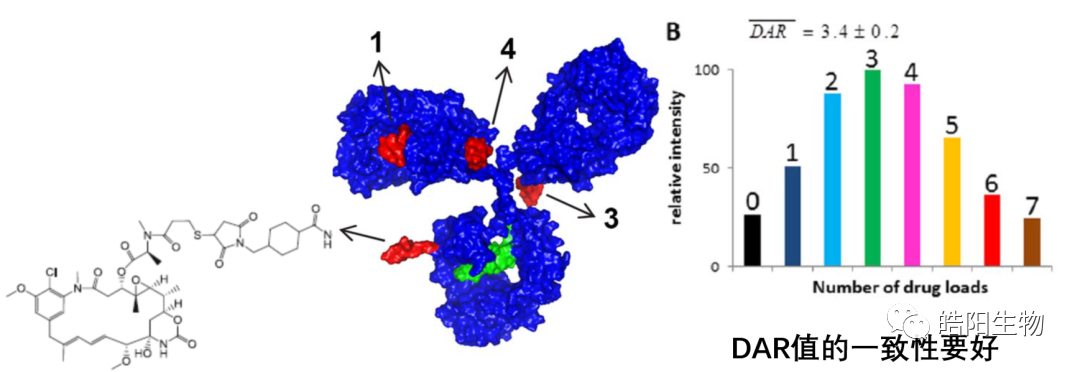
There are three types of antibody thiol coupling technologies, two of which belong to site-specific coupling technologies. The ADCs based on partially reduced thiol coupling of disulfide bonds have the key technical point of adjusting the amount of reducing agent to control the final DAR value, and the process is mature. Meanwhile, by fully reducing the four disulfide bonds of IgG1, eight thiols are obtained, which can bind with small molecules bearing maleimide functional groups to achieve ADCs with DAR values of 7-8. Compared to the previous technology, this coupling technology can achieve ADCs with relatively uniform DAR value components. The second site-specific coupling technology is bridging, which can increase the content of DAR4 by more than 30%, but compared to other thiol couplings, the number of chemical reaction steps will increase, and costs will increase accordingly. Combining these three coupling technologies, we can anticipate the development trend of coupling technology, where the regulation of DAR value has evolved from over 70 amino acid couplings with 1-8 small molecules to the current 4 disulfide bonds coupling with 1-8 small molecules and 2 sugar chains coupling with 2-4 small molecules, with more precise coupling sites and corresponding more complex chemical reactions, requiring higher precision control.
Generally, the physicochemical analysis and structural characterization of ADCs not only include the detection and analysis of characteristic parameters of individual antibodies, small molecule drugs, and linkers, but also include the detection of unique properties of ADC molecules such as Drug-Antibody Ratio (DAR), drug distribution, unlinked antibody ratio, and coupling sites. In this regard, this article comprehensively introduces the technical methods related to the physicochemical analysis and structural characterization of ADCs.
Table 1 Key Content of Structural Analysis and Quality Control of ADC Drugs

1. Size Exclusion Chromatography (SEC)
Size exclusion chromatography (SEC) is a technology that separates based on the size differences of protein molecules, which can be used to analyze the molecular weight and apparent molecular weight of ADCs and is the most commonly used method for studying molecular size variants.
When using SEC for analysis, a high ionic strength aqueous solution is usually required as the mobile phase to shield the interactions between molecules and the stationary phase. However, since the small molecules conjugated to ADCs usually have high hydrophobicity, using the mobile phase for monoclonal antibody SEC analysis may lead to broader peaks and lower resolution in the SEC results.
To improve the SEC results, a small amount of organic solvent can be added to the mobile phase, such as acetonitrile or isopropanol (Figure 1). Additionally, the type of ions in the mobile phase may significantly affect the separation effect of SEC technology for ADCs (Figure 2).
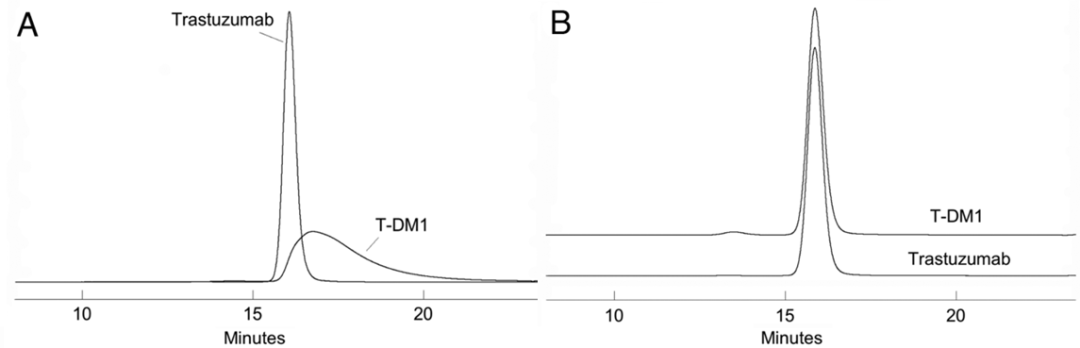
Figure 1 SEC Spectrum of mAb (Trastuzumab) and ADC (T-DM1) (A: No Organic Solvent; B: 15% Isopropanol Added)
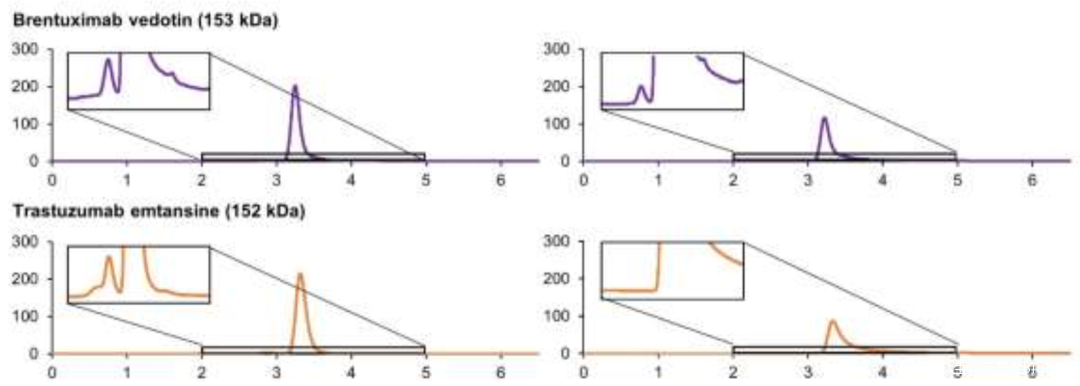
Figure 2 SEC Spectra of Two ADCs (Left: Potassium Phosphate; Right: Ammonium Acetate)
2. Hydrophobic Interaction Chromatography (HIC)
Hydrophobic interaction chromatography (HIC) separates based on the strength of hydrophobic interactions between different protein molecules and the stationary phase, maintaining the natural conformation of protein samples during the separation process. It is a typical and efficient non-denaturing analytical method that can be used to detect and calculate the DAR value and unlinked antibody molecular ratio of ADCs.
For HIC, both the stationary phase and the mobile phase are important factors affecting its separation effect. The factors related to the mobile phase include whether to add organic solvents, which organic solvents to add, and what concentration gradient of organic solvents to choose. Additionally, the nature of the stationary phase is also very important.
Taking the marketed ADC drug Kadcyla as an example, the amount of organic solvent added during the HIC separation process has a significant impact on the peak time and peak shape of different DAR value ADC molecules, and it also affects the final calculation results of the DAR value. As shown in Figure 3, when the organic solvent isopropanol (or acetonitrile) in mobile phase B is at 18%, it can better distinguish ADC molecules of different DAR values. Furthermore, compared to the commonly used linear gradient, setting the gradient of the mobile phase as a logarithmic gradient can yield better separation. This is because when the number of small molecule drugs conjugated is small, the increase in the number of small molecule drugs has a significant impact on the hydrophobicity of the ADC drug, while the difference in hydrophobicity between two different ADC molecules with DAR values of 6 and 8 is not significant, thus requiring a smaller organic solvent concentration gradient for better separation (Figure 4).
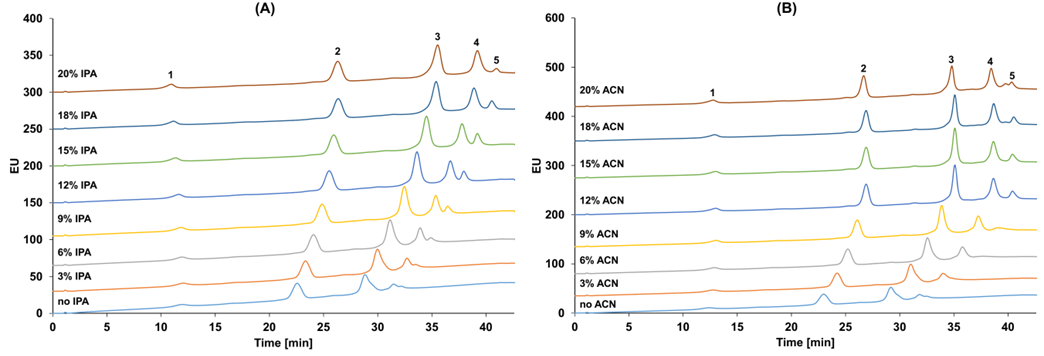
Figure 3 HIC Results of Kadcyla (1: DAR0; 2: DAR2; 3: DAR4; 4: DAR6; 5: DAR8). Separation Conditions: Mobile Phase A: 4.5 M NH4OAc; Mobile Phase B: 20 mM NH4OAc with Different Concentrations of IPA (A) and ACN (B).
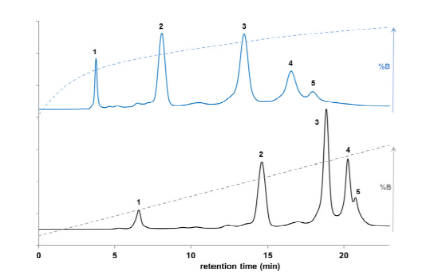
Figure 4 Comparison of Logarithmic and Linear Gradient HIC Separation Results of Kadcyla. Mobile Phase A: 4 M NaCl and 10 nM Phosphate Buffer; Mobile Phase B: 10 mM Phosphate Buffer and 8% Isopropanol.
3. Reversed-Phase Liquid Chromatography (RPLC)
RPLC is similar to HIC, as both aim to separate based on the hydrophobic interactions between protein molecules and the stationary phase.
The difference between the two is that RPLC typically has a stronger hydrophobic stationary phase; when analyzing ADCs with HIC, no sample pre-treatment is required, while RPLC analysis usually requires reducing the ADC molecules with TECP or DTT to achieve higher resolution and analyze key quality attributes (CQA) such as DAR values and coupling positions.
RPLC is widely used in the analysis of ADC molecules designed with Cys or site-specific coupling strategies. As shown in Figure 5, utilizing RPLC to analyze several ADCs with site-specific coupling, the coupling sites are LC-S7 (a) and HC-F404 (b), and it can be calculated that their DAR value is 2. For ADC drugs with random Cys coupling, the specific coupling sites can usually be analyzed by comparing RPLC results with those of SDS-PAGE (CE-SDS).

Figure 5 RPLC Results of Coupling Sites at LC-S7 (a) and HC-F404 (b) for Several ADC Drugs
4. Ion Exchange Chromatography (IEC)
Ion exchange chromatography (IEC) technology achieves separation based on the differences in the binding ability of different samples with the stationary phase at different pH mobile phases. It is a typical non-denaturing, charge heterogeneity analysis method, where salt ions and pH gradients significantly affect the separation results.
IEC is suitable for analyzing ADC drugs where small molecule conjugation has a significant impact on the antibody’s charge, such as lysine coupling; linkers with charged small molecules, etc.
5. Capillary Electrophoresis-Sodium Dodecyl Sulfate (CE-SDS)
Capillary electrophoresis-sodium dodecyl sulfate (CE-SDS) separates protein samples based on the molecular sieve effect, making it an efficient tool for studying the size heterogeneity of ADCs. In this method, since SDS can disrupt inter-chain hydrogen bonds, it is more suitable than SEC for routine analysis of protein fragments.
When analyzing ADC drugs using CE-SDS, method development should be based on the coupling methods and types of small molecules. Specifically:
Cys Coupled ADCs: Typically, CE-SDS results are compared with HIC, PRLC, etc. to obtain CQA information (Figure 6).
Lys Coupled ADCs: Analysis can be performed by comparing non-reduced CE-SDS and reduced CE-SDS results. It should be noted that this method is more suitable for ADCs with relatively large molecular weights. Moreover, compared to non-reduced CE-SDS, the reduced CE-SDS profiles have higher distinction for different DAR value molecules (Figure 7).

Figure 6 HIC (Non-Denaturing), CE-SDS (SDS Denaturing), RPLC (Reduced) Analysis Results of Cys Coupled ADCs

Figure 7 CE-SDS Results of Lys Coupled ADC Drugs
6. Capillary Isoelectric Focusing/Imaging Capillary Isoelectric Focusing Electrophoresis (cIEF/icIEF)
Capillary isoelectric focusing/imaging capillary isoelectric focusing electrophoresis (cIEF/icIEF) is a method that separates samples based on the differences in the isoelectric point (pI) of protein samples, serving as an effective tool for studying charge heterogeneity in ADCs.
Similar to IEF, cIEF/icIEF is also suitable for analyzing ADCs that undergo charge changes upon coupling. However, cIEF has a higher resolution than IEF and can provide characteristic profiles of ADC molecules, making it suitable for product release testing (Figure 8).

Figure 8 cIEF Results of Lys Coupled ADC Drugs (A: Fingerprint Profile; B: cIEF Profiles of ADC Drugs from Different Batches)
7. Mass Spectrometry-Based Analysis Methods (MS)
Mass spectrometry-based analysis methods can analyze the characteristics of ADCs at multiple scales, including intact molecules, subunits, and peptide fragments, making them the best tools for CQA analysis and consistency evaluation of ADC drugs.
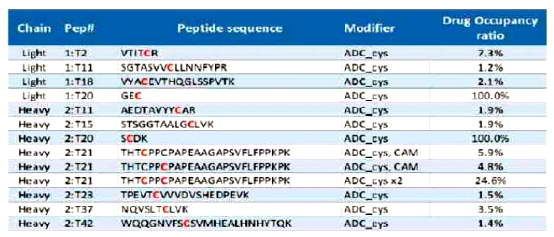
For different coupling methods, suitable MS analysis methods should be developed to achieve better results. For example, combining RPLC, SEC-HPLC, or capillary electrophoresis with mass spectrometry can be used to analyze and characterize ADC attributes such as Drug-Antibody Ratio (DAR), drug distribution, unlinked antibody ratio, and coupling sites (Figures 9-11 and Table 2).
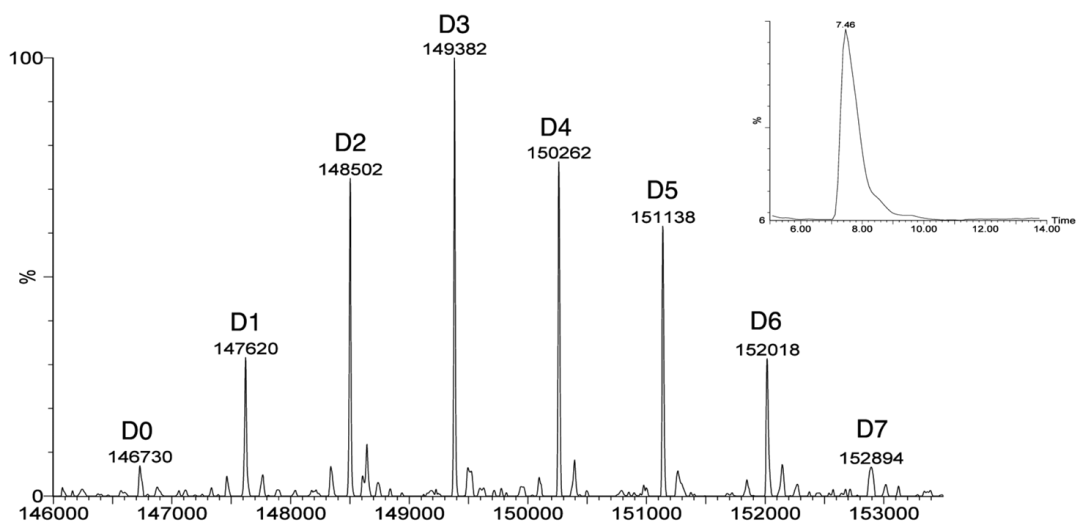
Figure 9 Determining ADC’s DAR Using RPLC-MS
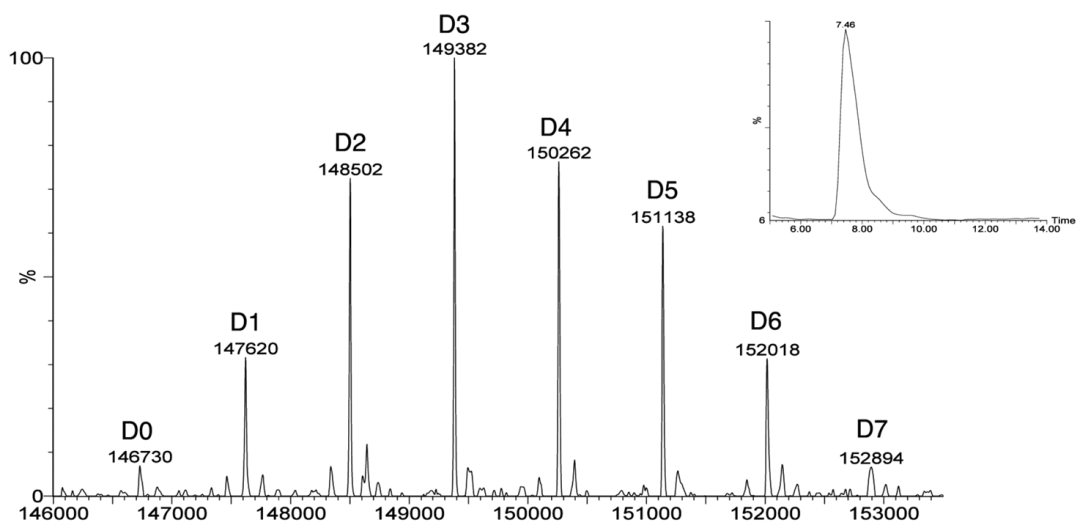
Figure 10 Analyzing ADC’s DAR and Drug Distribution Using SEC-MS

Figure 11 Analyzing ADC’s Drug Distribution by Comparing cIEF and MS Results
Table 2 Using MS Methods to Characterize ADC’s Coupling Sites and Ratios
6. Common Biological Analysis Methods for ADC Drugs
The unique structure of ADCs brings complex toxicological characteristics. ADC drugs benefit from their conjugated structural features, and compared to traditional anti-tumor drugs, ADCs have longer circulation times in serum, stronger therapeutic efficacy, higher specificity for tumor cells, weaker toxicity to non-target cells, and significantly reduced systemic toxicity. However, the complex structure brings unique toxicological characteristics. Due to the presence of large molecular monoclonal antibody structures, the in vivo distribution of ADCs is mainly limited to plasma, extracellular fluid, and lymphatic tissues, exhibiting pharmacokinetic characteristics similar to large molecular antibodies, such as low clearance rates, long half-lives, low volume of distribution, extremely poor oral bioavailability, nonlinear distribution and elimination, and the generation of immunogenicity. However, due to the presence of small molecules and linkers, ADCs have more diverse degradation pathways and toxicity compared to large molecular drugs, which also leads to anti-drug antibodies (ADA) originating from different sources than naked antibodies.
1. Total Antibody (Bound Antibody and Unbound Antibody) Analysis: ELISA, Electrochemiluminescence Immunoassay (ECLA)
In non-clinical PK and TK studies of ADC drugs, the antibodies selected for detection generally fall into two categories: a) polyclonal antibodies that bind to the non-variable region of the antibody (anti-Fc antibodies); b) anti-human IgG antibodies;
In clinical trials of ADC drugs, recombinant antigens, unique-type antibodies, or anti-complementarity determining region (CDR) antibodies can be used to reduce non-specific binding and minimize serum protein background.
Due to the biotransformation of ADC drugs in vivo, the changes in DAR may still be affected, so the ADC mixtures at different PK sampling time points exhibit dynamic changes. Due to the dynamic changes of ADC mixtures; when ADC drugs have high DAR, it may lead to the detection reagents being unable to bind to the ADC drug. Therefore, the reference standards used for quantitative detection in ELISA may not fully reflect the levels of the analytes in the samples. For all total antibody detection modes for ADCs used in non-clinical and clinical development, it is important to ensure that all expected DARs in vivo are accurately quantified as total antibody concentrations.
2. Coupled Antibody ADC Analysis: ELISA, Electrochemiluminescence Immunoassay (ECLA)

In the PK analysis of coupled antibodies, antibodies against small molecules are usually used as coating agents, and the detection of antibodies remains consistent with total antibody analysis. ELISA is typically used to determine the antibody conjugates in serum. LBA methods can analyze all DAR analytes but do not include naked antibodies in the reference standards or naked antibodies resulting from complete dissociation in vivo. However, the drawback of this analyte is that it cannot provide direct information on drug load. This is because the analytical mode can generate signal values when ADC drug DAR ≥ 1 due to the binding of enzyme-linked immunosorbent reagents. Unlike total antibody analysis, where theoretical binding with high DAR may be poor due to steric hindrance, in antibody conjugate ELISA, binding with low DAR (such as DAR=1) may also be poor due to low affinity. If low DAR cannot be accurately detected in the trial, the PK profile will overestimate the amount of drug that is completely dissociated.
3. Small Molecule Cytotoxin Analysis: LC-MS/MS
For free small molecule drugs in plasma and their related metabolites bearing drug structures, protein precipitation and/or solid-phase extraction are required to remove plasma proteins prior to LC-MS/MS quantification. If quantitative analysis of drug-related metabolites is needed, these in vivo metabolites must first be identified and their standards prepared. Once the structures of the main metabolites are determined and stable isotope-labeled internal standards or surrogate molecules are synthesized, traditional small molecule LC-MS/MS analysis methods can be applied to non-clinical and clinical studies of small molecule metabolites.
In some cases, if the drug has an active part, such as sulfonamide, the drug released from ADC may dimerize or covalently bind to plasma proteins. In such cases, additional sample preparation steps are needed to chemically reduce plasma samples to release any dimerized drugs or drugs covalently bound to plasma proteins. Additionally, for ADCs, the stability of analytes in the presence of ADC needs to be evaluated. This is important because, in addition to the concentration of drug analytes decreasing due to instability during storage, the drug concentration may also increase due to the release of drugs from ADC during storage.
4. Immunogenicity Analysis—Anti-Drug Antibodies (ADA), Neutralizing Antibodies (NAb)
Like all biopharmaceuticals, ADCs have the potential to induce immune responses. ADCs may also lead to immunogenicity in humans, producing corresponding anti-drug antibodies (ADA), which can affect PK, PD, and safety; components of ADCs, such as antibodies and linkers, may induce immunogenicity. Besides the immunogenicity common to all biopharmaceuticals, ADCs have unique aspects; when ADC molecules or ADC fragments bearing drug molecules combine with ADA, the immune complexes may deliver cytotoxic drugs to unintended locations, leading to safety risks.

The analysis process for ADA includes the following four levels: screening, confirmation, titer, and neutralization experiments.
a. Screening Experiment: Based on the screening threshold, biological samples are initially screened (with a 5% false positive rate); samples above the threshold are considered potentially positive, and those below are negative samples;
b. Confirmation Experiment: Positive samples identified in the screening undergo immune confirmation to verify the specificity of the antibodies to the drugs; potential positive samples can also undergo drug suppression tests, also known as competitive inhibition tests; the sample is determined to be positive based on the inhibition rate before and after drug addition;
c. Titer Experiment: Positive ADA samples undergo titer testing to analyze the strength of the antibodies, diluting the positive samples until the diluted sample equals the screening threshold, thus determining the antibody titer;
d. Neutralization Experiment: Neutralizing antibodies (NAb) are a special type of ADA that block the product from reaching its target or interfere with receptor/ligand binding, thereby interfering with the in vivo activity of the drug. Currently, there are two approaches for immunogenicity analysis: 1) Cell-based assay: using in vitro functional tests based on cells to evaluate neutralizing antibodies (NAb), or 2) Non-Cell-based assay: for certain biopharmaceuticals, competitive ligand binding assays (LBA) can be selected to evaluate neutralizing antibodies (NAb) based on their mechanisms of action.
7. Summary by the Author
In the past few decades, ADC drugs have made significant progress in conjugation chemistry, production, and product stability.
Compared to naked antibodies, the inherent complexity of ADC drug molecules presents significant challenges in developing analytical methods. To better control the quality, stability, and safety of ADC drugs, a more comprehensive and in-depth understanding of the characteristics of ADC drug molecules is needed, thus requiring the selection of appropriate tools for multi-dimensional detection and analysis of ADC molecules based on conjugation methods and types of small molecule drugs.
References
1. Hongcheng Liu, Kimberly May, Disulfide bond structures of IgG molecules, mAbs, 2012,4,17-23.
2. James McNulty, Venkatesan Krishnamoorthy, Dino Amoroso et.al, Tris(3-hydroxypropyl)phosphine (THPP): A mild, air-stable reagent for the rapid, reductive cleavage of small-molecule disulfides, Bioorganic & Medicinal Chemistry Letters,2015, 25,4114–4117.
3. Takashi Nakada, Takeshi Masuda, Hiroyuki Naito et.al, Novel antibody drug conjugates containing exatecan derivative-based cytotoxic payloads, Bioorganic & Medicinal Chemistry Letters, Bioorg Med Chem Lett., 2016,26,1542-1545.
4. Abigail R. Hanby, Stephen J. Walsh, Andrew J. Counsell et.al, Antibody dual-functionalisation enabled through a modular divinylpyrimidine disulfide rebridging strategy, Chem. Commun., 2022, 58, 9401.
5. Yuuri Hashimoto, Kumiko Koyama, Yasuki Kamai et.al, A novel HER3-targeting antibody-Drug conjugate, U3-1402, exhibits potent therapeutic efficacy through the delivery of cytotoxic payload by efficient internalization, Clin Cancer Res. 2019,25,7151-7161.
6. Wagh, A.; Song, H.; Zeng, M.; Tao, L.; Das, T. K., Challenges and new frontiers in analytical characterization of antibody-drug conjugates. mAbs 2018,10 (2), 222-243.
7. Xu, Y.; Jiang, G.; Tran, C.; Li, X.; Heibeck, T. H.; Masikat, M. R.; Cai, Q.; Steiner, A. R.; Sato, A. K.; Hallam, T. J.; Yin, G., RP-HPLC DAR Characterization of Site-Specific Antibody Drug Conjugates Produced in a Cell-Free Expression System. Organic Process Research & Development 2016,20 (6), 1034-1043.
8. Wiggins, B.; Liu-Shin, L.; Yamaguchi, H.; Ratnaswamy, G., Characterization of Cysteine-Linked Conjugation Profiles of Immunoglobulin G1 and Immunoglobulin G2 Antibody–Drug Conjugates. Journal of Pharmaceutical Sciences 2015,104 (4), 1362-1372.
9. Luo, Q.; Chung, H. H.; Borths, C.; Janson, M.; Wen, J.; Joubert, M. K.; Wypych, J., Structural Characterization of a Monoclonal Antibody–Maytansinoid Immunoconjugate. Analytical Chemistry 2016,88 (1), 695-702.
10. Rodriguez-Aller, M.; Guillarme, D.; Beck, A.; Fekete, S., Practical method development for the separation of monoclonal antibodies and antibody-drug-conjugate species in hydrophobic interaction chromatography, part 1: optimization of the mobile phase. Journal of Pharmaceutical and Biomedical Analysis 2016,118, 393-403.
11. Bobaly, B.; Beck, A.; Veuthey, J.-L.; Guillarme, D.; Fekete, S., Impact of organic modifier and temperature on protein denaturation in hydrophobic interaction chromatography. Journal of Pharmaceutical and Biomedical Analysis 2016,131, 124-132.
12. Goyon, A.; D’Atri, V.; Colas, O.; Fekete, S.; Beck, A.; Guillarme, D., Characterization of 30 therapeutic antibodies and related products by size exclusion chromatography: Feasibility assessment for future mass spectrometry hyphenation. Journal of Chromatography B 2017,1065-1066, 35-43.
13. Wakankar, A.; Chen, Y.; Gokarn, Y.; Jacobson, F. S., Analytical methods for physicochemical characterization of antibody drug conjugates. mAbs 2011,3 (2), 161-172.