Since 2000, the ADC field has gradually started to be pushed to market, and in recent years, it has emerged rapidly in the field of cancer treatment, leading to intense competition. To differentiate themselves, some companies are beginning to develop ADCs for non-oncology areas. Although the number of related ADCs is extremely limited and most are still in preclinical stages, this represents a new approach. Next, we will introduce some of these promising areas.
Bacterial Infections: ADCs can target specific bacterial pathogens and deliver antibiotics directly to the bacteria, which may help reduce antibiotic resistance and protect beneficial microbiota.
Viral Infections: ADCs can be designed to target viral proteins, providing a new approach for treating viral infections such as HIV and hepatitis.
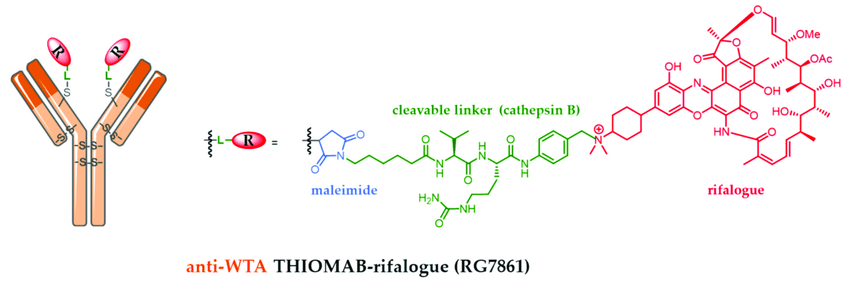
For example, Genentech’s RG-7861 is an Anti-S. aureus AAC, consisting of a THIOMAB™ IgG1 that recognizes Staphylococcus aureus linked to a rifampicin-class antibiotic. After the antibody binds to the golden staph, it enters phagocytic cells, where the rifampicin antibiotic is released to kill the staph inside the phagocytic cells. It is currently in phase I clinical trials.
RG-7861 Related Information
Autoimmune diseases have become the most concentrated area of non-cancer ADC indications, with the highest number of projects in rheumatoid arthritis. Especially, the leading company in the autoimmune field, AbbVie, is also laying out ADCs in this area, such as ABBV-3373. ABBV-3373 consists of adalimumab and a novel glucocorticoid receptor modulator (GRM), aiming to directly deliver the effective payload GRM to activated immune cells expressing TNFα, thereby modulating TNF-mediated inflammatory pathways.
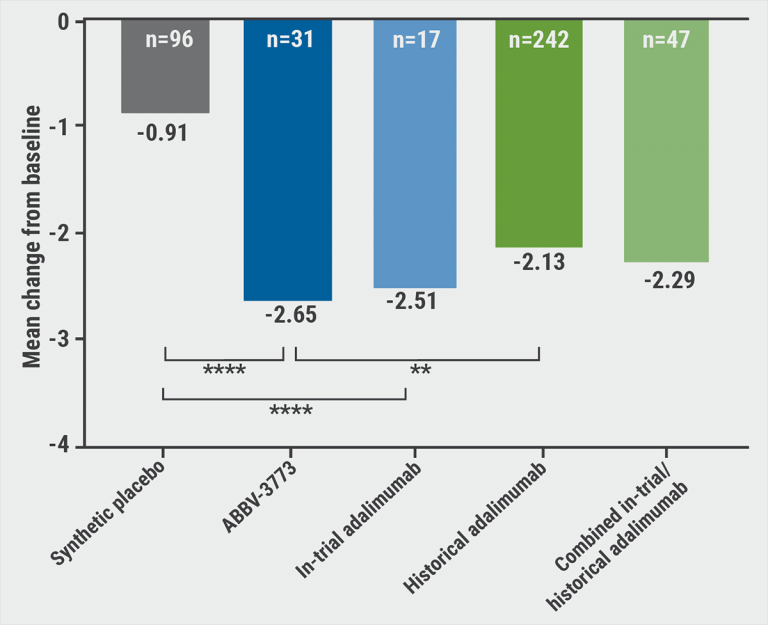
Clinical Results of ABBV-3373
In June 2020, AbbVie announced positive results from the phase II M16-560 study of ABBV-3373 for treating moderate to severe rheumatoid arthritis (RA). Compared to the pre-specified historical average of adalimumab (-2.13), the ABBV-3373 treatment group showed a greater change from baseline to week 12 in the primary endpoint DAS28-CRP (-2.65, p=0.022). A second statistical comparison based on Bayesian analysis indicated that ABBV-3373 had a 90% probability of showing greater improvement in DAS28-CRP from baseline to week 12 compared to adalimumab, which combined trial and historical data. In terms of safety, the incidence of adverse events in the ABBV-3373 treatment group was lower than that in the adalimumab treatment group (35% vs 71%).
Although AbbVie has currently terminated the ABBV-3373 project, this has provided ideas for other companies. For example, domestic companies are also actively laying out:
-
On April 7, 2024, Hengrui Medicine’s CD40 ADC patent was disclosed. This CD40 ADC mainly explores applications for treating autoimmune diseases.
-
Ying’en Bio’s BDCA2 ADC patent was disclosed. It uses glucocorticoids as payloads for treating autoimmune diseases.
In the context of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease, the number of studies related to ADCs is on the rise.
-
Alzheimer’s Disease: ADCs targeting amyloid β plaques or tau protein aggregates could provide new treatment options for neurodegenerative diseases.
-
Multiple Sclerosis: ADCs can selectively target and modulate pathogenic immune cells or inflammatory processes in the central nervous system.
At the Alzheimer’s Association International Conference (AAIC 2024), AC Immune announced a new class of candidate drugs for neurodegenerative diseases called Morphomer antibody-drug conjugates (morADC).
morADC can target important targets including β-amyloid (Aβ), Tau, and α-synuclein, while allowing single or dual-targeting strategies (for example, combining anti-Aβ antibodies with anti-Tau small molecules), providing combination therapy in a single formulation.
morADC Improves Drug Penetration Across the Blood-Brain Barrier
In preclinical studies, morADC was shown to cross the blood-brain barrier five times better than traditional antibodies, significantly improving drug exposure levels in the central nervous system.
Studies have found that morADC drugs targeting both Aβ and Tau proteins (monoclonal antibody targeting Aβ, Morphomer targeting Tau) have three times the inhibitory activity against Aβ42 compared to corresponding antibodies and fifteen times the inhibitory activity against Tau compared to corresponding Morphomers. This also provides ideas for treating neurological diseases.
GLP-1R and its ligand GLP1 are highly validated targets for obesity and type 2 diabetes, which goes without saying. Currently, drug development targeting this target mainly focuses on peptide and small molecule development, with only peptide drugs already on the market.
However, these marketed peptide drugs have certain drawbacks, such as requiring weekly or more frequent dosing. To improve this drawback, developing GLP-1R agonist antibodies is a potential strategy, but there are currently no GLP-1R direct agonist antibodies.
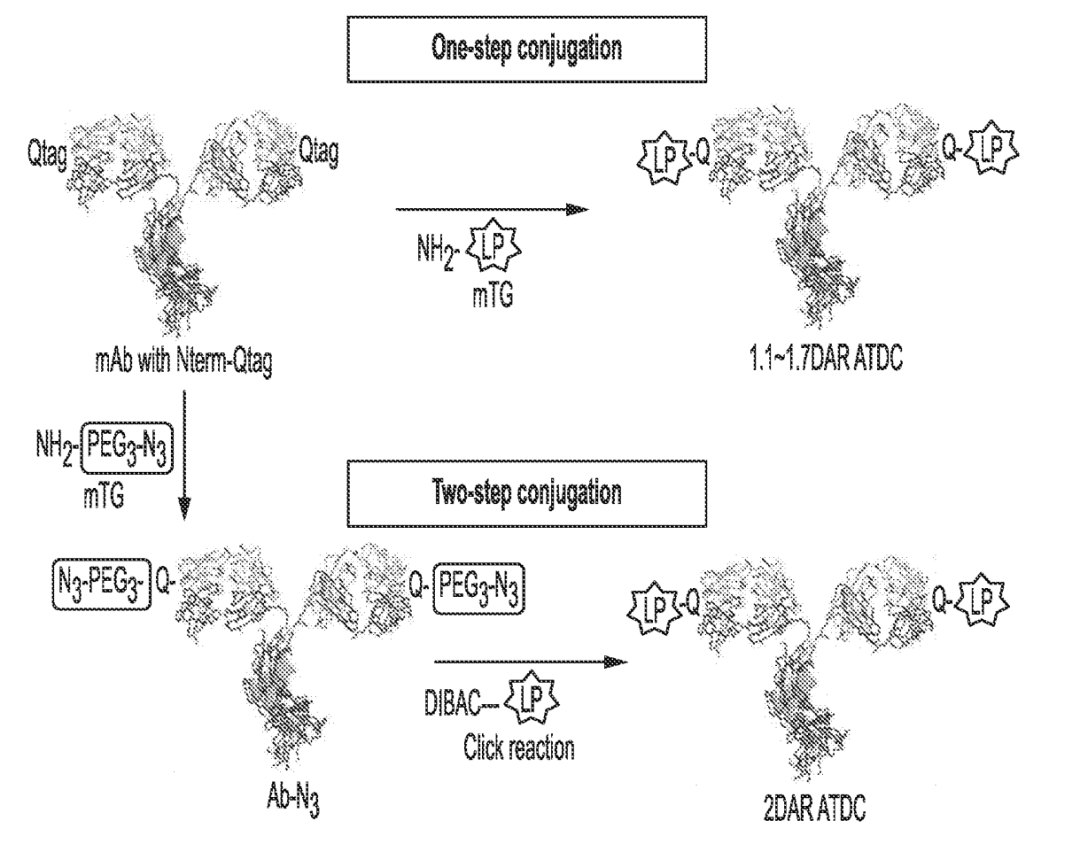
Regeneron has provided a new solution: an antibody-drug conjugate combining an antibody or its antigen-binding fragment that specifically targets the extracellular domain of GLP1R with a functional GLP1 peptide mimetic that activates GLP1R. Regeneron has coined a new term for this technology: antibody-tethered drug conjugates (ATDCs).
Unlike ADCs carrying cytotoxic drugs, ATDCs do not require the linker to break. By linking the GLP-1R antibody, the drug’s affinity is greatly increased while also increasing the drug’s relative molecular weight, enhancing the stability and half-life of the GLP-1 peptide mimetic. In patent WO2023173132A1 disclosed on September 14, 2023, ATDC can maintain weight loss effects for at least 4 weeks after a single dose.
BalancingR&D Investment Between Oncology and Other Therapeutic Areas
The prioritization of investments is based on factors such as market demand, scientific feasibility, regulatory environment, competitive landscape, potential for breakthrough therapies, strategic fit, partnerships, and financial considerations.
Due to the severity of oncological diseases, oncology typically has accelerated approval pathways, attracting R&D investments. Accelerated programs such as the FDA’s breakthrough therapy designation and fast track can shorten development times and reduce risks. Clear and supportive regulatory pathways for non-oncological diseases can also encourage investments in these areas.
The fierce competition in the oncology field may prompt companies to seek opportunities in less competitive areas, assessing the number of competitors and the status of competing therapies. Therapeutic areas with fewer competitors or novel targets may be attractive, providing companies with opportunities to establish leadership and differentiated products.
1.Antibody- Drug Conjugates: The Last Decade
2.https://www.adcreview.com/drugmap/dsta4637s/
https://conferences.medicom-publishers.com/specialisation/rheumatology/eular-2021/abbv-3373-a-potential-new-therapeutic-agent-for-ra/
3.Exploring the potential of ADCs beyond oncology. By Mike Ward
4.The Next Trend for GLP-1, Small Molecules? ATDC? Drug Intelligence Network
5.New Directions in Antibody-Drug Conjugate Development.Pharmaceutical Magic Cube
Scan the WeChat QR code to add the editor of the Biologics Circle, eligible individuals can join
Please specify: Name + Research Direction!
All articles reprinted by this public account are for the purpose of conveying more information, and the sources and authors are clearly indicated. Media or individuals who do not wish to be reprinted can contact us ([email protected]), and we will immediately delete the content. All articles only represent the author’s views and do not represent the position of this site.