Antibody-drug conjugates (ADC) are comprised of monoclonal antibodies that target specific antigens linked to small molecule cytotoxic drugs via a linker, combining the powerful killing effects of traditional small molecule chemotherapy with the tumor-targeting properties of antibody drugs. Since the first ADC (Gemtuzumab-ozogamicin (trade name: Mylotarg)) was approved for the treatment of CD33-positive acute myeloid leukemia, several ADCs have been developed for cancer treatment.
The entire development process of ADCs, from selecting the appropriate antibody to the final product, is a daunting and challenging task. Clinical pharmacology is one of the most important tools in drug development, utilizing this tool helps to find the optimal dosing of products, thereby maintaining the safety and efficacy of products in patient populations. Unlike other small or large molecules that typically measure only one component and/or metabolite for pharmacokinetic analysis, ADCs require the measurement of multiple components to characterize their PK properties. Therefore, a deep understanding of the clinical pharmacology of ADCs is crucial for selecting safe and effective doses in patient populations.
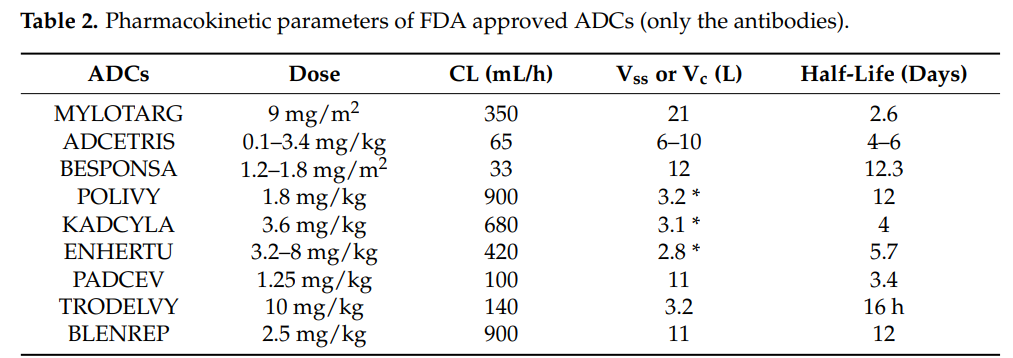
Pharmacokinetics is an indispensable part of clinical pharmacology and modern drug development processes. The primary aim of pharmacokinetics studies is to obtain information about drug absorption, volume of distribution, clearance rate, half-life, accumulation after multiple dosing, and the effects of various disease states, as well as age, weight, and sex on drug pharmacokinetics. These pharmacokinetic parameters can be used to design the optimal dosing regimen for patients.
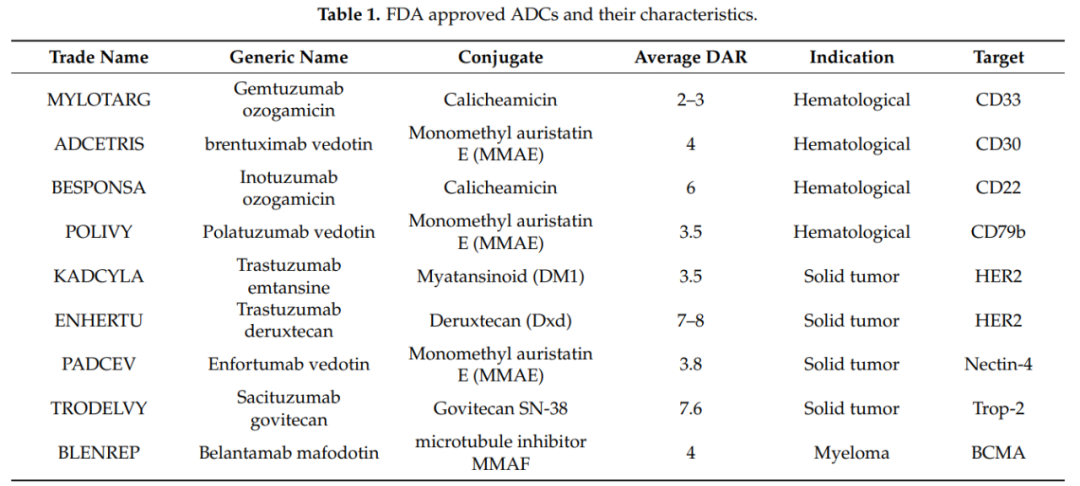
It should be recognized that, unlike small molecules and therapeutic proteins (antibodies or fusion proteins), the PK of ADCs is very complex because ADCs consist of several components. Not only the PK of the monoclonal antibody must be considered, but also the PK of the cytotoxic molecule and the physicochemical properties of the conjugates. Since the molecular weight of the monoclonal antibody accounts for over 90%, the PK of the different components of the ADC is greatly influenced by its PK. The total antibody (ADC+mAb) PK characteristics provide the best assessment of ADC stability and integrity. The conjugate and the conjugation site also play an important role in maintaining the stability and PK of ADCs. The table below lists the FDA-approved ADCs and their PK characteristics.
Generally, four processes are involved in the body after administration. These processes are absorption, distribution, metabolism, and elimination.
Absorption
Most antibodies are usually administered via intravenous injection or infusion, and antibodies can also be administered subcutaneously (SC). However, for ADCs, the current administration route is intravenous injection or infusion. Due to the response to cytotoxic payloads and the local deposition of cytotoxic substances, SC administration may not be suitable for ADCs.
Distribution
The distribution of drugs in the body can be described by the volume of distribution. Due to their size and polarity, the distribution of antibodies and ADCs is usually limited to the vascular and interstitial spaces.
The initial distribution of ADCs is generally limited to the blood vessels, with a volume of distribution generally equal to blood volume. Subsequently, ADCs can distribute to interstitial spaces. Furthermore, the distribution of ADCs is also influenced by the expression of target antigens and endocytosis.
The distribution and accumulation of ADCs in the same tissue can lead to adverse (toxic) pharmacological effects due to the release of cytotoxic drugs or metabolites after the uptake of ADCs.
Metabolism
The degradation/metabolism process of ADCs in the body includes the metabolic processes of antibodies and small molecule drugs. Before reaching tumor cells, ADCs release effector molecules either intracellularly (non-cleavable linker) or in the circulatory system (cleavable linker), and unbound antibodies and antibody fragments follow the metabolic pathway of antibodies, producing amino acids through enzymatic hydrolysis, which are reused by the body.
The free small molecule drugs and/or small molecule drug metabolites linked to amino acid residues that may form after the cleavage or degradation of ADCs will further undergo hepatic CYP450 enzyme metabolism and may also lead to potential drug-drug interactions.
In addition to the properties of the ADC itself, the expression of antigens, receptor/cell density, FcRn-mediated recycling, interactions with Fcγ, receptor-mediated endocytosis, and immunogenicity can all affect the degradation and metabolism of ADCs.
Elimination
ADCs are eliminated through degradation and excretion. ADCs can enter lysosomes via specific pathways that bind to targets, undergo degradation, and release small molecule drugs for clearance from the body; they can also be cleared through non-specific pinocytosis, which involves the recycling process mediated by the neonatal receptor (FcRn).
ADCs, antibodies, larger peptides, and amino acid fragments cannot be excreted through glomerular filtration but are reabsorbed and utilized in the form of amino acids. Free small molecule drugs, smaller peptides, and small molecule drugs linked to amino acids, as well as smaller antibody fragments, can be excreted through glomerular filtration. Additionally, small molecule drugs and metabolites can also be eliminated via enzymatic metabolism or excreted into feces through transporters.
ADCs consist of several components, and various analytical methods are required to characterize the PK characteristics of these components, as described below:
-
ELISA immunoassays to determine the kinetics of conjugates and total antibodies;
-
TFC-MS/MS for quantitative analysis of free drugs/metabolites;
-
High-resolution mass spectrometry for in vivo drug-antibody ratio (DAR) analysis.
In addition, two types of ELISA immunoassays are used to quantitatively measure the analytes of ADCs: the first type measures total antibodies, i.e., ADCs with DAR greater than or equal to zero. The second type measures drug-conjugated antibodies, defined as ADCs with DAR greater than or equal to one.
Other analytical methods include size-exclusion chromatography (SEC) and hydrophobic interaction chromatography (HIC). SEC is the most commonly used liquid chromatography (LC) technique for determining the quantity of antibody aggregates, and this technique can also be applied to ADCs. Although HIC is a traditional technique for protein separation, purification, and characterization, it is now being applied to the characterization and analysis of ADCs.
The cytotoxic payloads of ADCs should possess the following characteristics:
-
The cytotoxic payload should have appropriate lipophilicity.
-
The target of the payload should be located inside the cell.
-
The payload should be small in size, lack immunogenicity, and be soluble in aqueous buffers to facilitate easy conjugation.
-
The payload should be stable in the bloodstream.
Currently, commonly used cytotoxic drug effect molecules include microtubule inhibitors (such as: auristatins, maytansinoids), DNA damaging agents (such as calicheamicin, duocarmycins, anthracyclines, pyrrolobenzodiazepine dimers), and DNA transcription inhibitors (Amatoxin and Quinoline alkaloid (SN-38)). Several ADC drugs that have been approved for market use employ six different small molecule drugs, of which three ADC drugs use MMAE as the conjugated drug, two drugs use Calicheamicin as the conjugated drug, and others successfully applied include MMAF, DM1, SN-38, and Dxd.
The drug-antibody ratio (DAR) refers to the average number of payload molecules attached to a single monoclonal antibody, usually between 2 and 4 molecules. In rare cases, using hydrophilic linkers, a DAR of up to 8 can be safely achieved, as seen in Enhertus and Trodelvys. DAR is crucial for assessing the efficacy of ADCs, and additionally, DAR may affect the drug’s stability in circulation, PK, and toxicity of ADCs.
Studies have shown that ADCs with a high DAR (7 to 14) clear faster and have reduced in vivo efficacy compared to ADCs with a DAR value < 6. The DAR value and its effects on stability and PK also depend on the conjugation site and the size of the linker.
Lysine or cysteine is typically modified to produce ADCs. Lysine is one of the most commonly used amino acid residues to connect substrates and antibodies, and lysine is usually present on the surface of antibodies, making it easy to conjugate. Mylotargs, Kadcylas, and Besponsas all utilize lysine bioconjugation technology.
Other amino acids, such as cysteine and tyrosine, can also be modified, with maleimide-modified cysteine being used to synthesize ADCs like Adcetriss, Polivys, Padcevs, Enhertus, Trodelvys, and Blenreps.
Linkers are an indispensable part of ADCs, determining the drug release mechanism, PK, therapeutic index, and safety of ADCs. Early ADC linkers were chemically unstable, such as disulfides and hydrazones. These linkers were unstable in circulation and had short half-lives, generally one to two days. The latest generation of linkers is more stable in circulation, such as peptide and glucuronic acid linkers. The two most common types of linkers are as follows:
Cleavable Linkers
Cleavable linkers are sensitive to the intracellular environment and release free effector molecules and antibodies through degradation and dissociation in the intracellular environment, such as acid-cleavable linkers and protease-cleavable linkers. They are generally stable in the bloodstream but can rapidly cleave in low pH and protease-rich lysosomal environments, releasing effector molecules. Additionally, if the effector molecules can cross membranes, they may eliminate tumors through potential bystander effects.
Non-Cleavable Linkers
Non-cleavable linkers are a new generation of linkers that provide better plasma stability compared to cleavable linkers. Since non-cleavable linkers can offer greater stability and tolerability than cleavable linkers, these linkers reduce off-target toxicity and provide a larger therapeutic window.
In 11 clinical trials targeting 8 ADCs, the baseline incidence of ADAs ranged from 1.4% to 8.1%, and the incidence of post-baseline ADAs ranged from 0-35.8%, values that fall within the range of therapeutic monoclonal antibodies. Overall, the ADA incidence of ADCs is lower in patients targeting hematological malignancies than in those targeting solid tumors; most ADAs are directed against the monoclonal antibody domain of ADCs. Moreover, in most patients, the semi-antigenic structure of these ADCs does not pose a greater risk of immune responses compared to therapeutic monoclonal antibodies.
The application of modeling approaches can integrate PK, efficacy, and safety data to meet the needs of different stages of ADC drug development, such as target selection, antibody affinity, linker stability, animal-to-human extrapolation, dose selection and adjustment, exposure-response relationships, DDI studies, etc. Due to the multiple clearance pathways of ADCs (dissociation and degradation) and the complex PK characteristics of various analytes, their kinetic models are also quite complex.
Different models have different applications, such as two-compartment models and PBPK models can describe the stability characteristics of ADCs using clearance rates, dissociation, and metabolic rates. Currently, non-compartmental models, population pharmacokinetic models, mechanism-based models, and physiology-based models are all being applied in ADC pharmacokinetic research.
In the development process of ADC drugs, clinical pharmacology plays a very important role. Through continuously evolving bioanalytical techniques, a comprehensive elucidation of the PK/PD characteristics of ADC drugs is crucial for promoting the development of less toxic and more effective ADC drugs. ADC drugs are expected to show even greater advantages in the field of cancer treatment.
References:
1. Clinical Pharmacology of Antibody-Drug Conjugates. Antibodies (Basel). 2021 May 21;10(2):20.
Training Recommendations
Shanghai Jiao Tong University – Advanced Training Course on New Drug Clinical Trials


All articles reproduced by this public account are for the purpose of conveying more information and are clearly marked with sources and authors. Media or individuals who do not wish to be reproduced can contact us ([email protected]), and we will immediately delete them. All articles represent the author’s views and do not represent the position of this site.

