Follow Xiaobai Learning Medicine As of now, 17 ADC drugs have been approved worldwide, of which 5 ADCs are coupled with lysine.
Table 1 Summary of ADC Drugs Coupled with Lysine Approved
|
Product Name |
Company |
Target |
DAR |
Toxin |
Linker |
Antibody Type |
Payload Type |
|
Mylotarg |
Pfizer |
CD33 |
2~3 |
Calicheamicin |
Hydrazone Link (Acid-labile) |
IG4 |
DNA Damaging Agent |
|
Kadcyla |
Roche |
Her2 |
3.5 |
DM1 |
SMCC (Non-cleavable) |
IG1 |
Microtubule Inhibitor |
|
Bseponsa |
Pfizer |
CD22 |
5~7 |
Calicheamicin |
Hydrazone Link (Acid-labile) |
IG4 |
DNA Damaging Agent |
|
Akalux |
Rakuten |
EGFR |
NA |
IRDye700DX |
Linear Alkyl/Aryloxy Linker (Non-cleavable) |
IG1 |
Photoimmunotherapy |
|
ELAHERE |
ImmunoGen |
FRa |
3.4 |
DM4 |
Sulfo-SPDB (Reducible) |
IG1 |
Microtubule Inhibitor |
1. Introduction to Kadcyla
Kadcyla: An ADC drug using the non-cleavable linker SMCC
Linker: SMCC (N-succinimidyl-4-(maleimidomethyl) cyclohexane-1-carboxylate) is a classic non-cleavable linker
Payload: DM1 (effectively inhibits the polymerization of microtubules, thereby blocking the mitotic process of cells, ultimately leading to apoptosis)
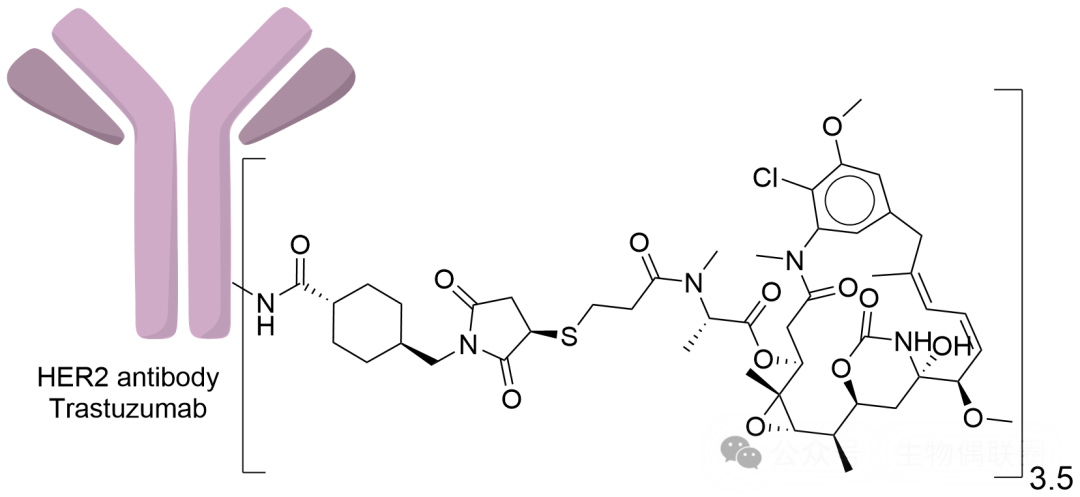
Figure 1 Kadcyla Structural Diagram
Table 2 Summary of Kadcyla Drug Information
|
Product Name |
Kadcyla |
|
Generic Name |
ado-trastuzumab emtansine |
|
Manufacturer |
Roche, Switzerland |
|
Indication |
HER2-positive breast cancer (for treatment of HER2-positive metastatic breast cancer patients who have previously received trastuzumab and taxane chemotherapy) |
|
Target |
HER2 |
|
Dosage Form |
Lyophilized Powder |
|
Specifications |
100mg, 160mg/vial – 20mg/mL |
|
Buffer Type |
Buffer Salt: 10mM Succinic Acid Excipient: 60mg/mL Sucrose Surfactant: 0.2mg/mL Polysorbate 20, pH 5.0 |
2. Introduction to Mylotarg
Mylotarg:ADC drug using acid-labile linker hydrazone
Linker: Hydrazone (C=N-N) bond is acid-sensitive (butyric acid ester bond connects to the antibody part)
Payload: Calicheamicin (disulfide bond (S-S) linkage), when the drug is internalized by target cells, the hydrazone bond breaks in the low pH environment while the disulfide bond is reduced by cytoplasmic glutathione reductase, releasing calicheamicin (which breaks DNA double strands, thereby inducing apoptosis in tumor cells).
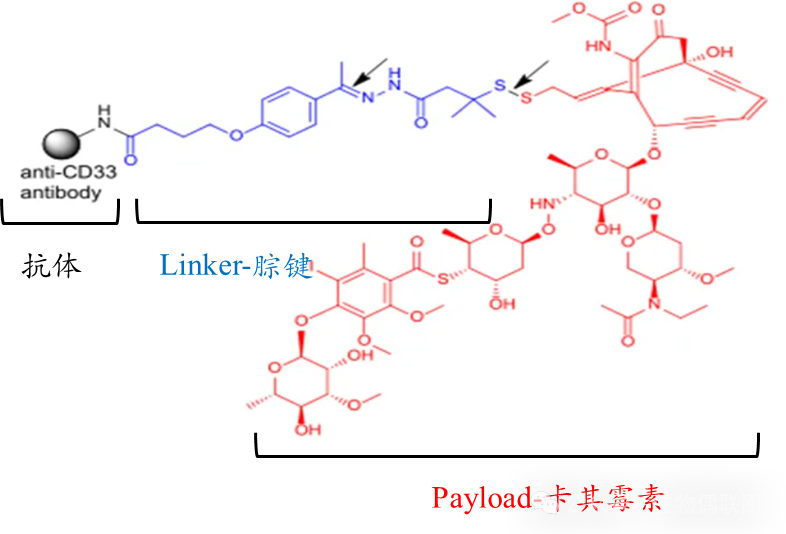
Figure 2 MylotargStructural Diagram
Table 3 Summary of Mylotarg Drug Information
|
Product Name |
Mylotarg |
|
Generic Name |
gemtuzumab ozogamicin |
|
Manufacturer |
Pfizer, USA |
|
Indication |
CD33-positive acute myeloid leukemia |
|
Target |
CD33 |
|
Dosage Form |
Lyophilized Powder |
|
Specifications |
4.5mg/vial – 1mg/mL |
|
Buffer Type |
Buffer Salt: 5mM Phosphate Excipient: 14mg/mL Sucrose, 8mg/mL Glucose 40 Surfactant: None, pH 7.4 |
3. BesponsaIntroduction
Besponsa: ADC drug using acid-labile linker hydrazone bond
Linker: Hydrazone (C=N-N) bond is acid-sensitive (butyric acid ester bond connects to the antibody part)
Payload: Calicheamicin (disulfide bond (S-S) linkage), when the drug is internalized by target cells, the hydrazone bond breaks in the low pH environment while the disulfide bond is reduced by cytoplasmic glutathione reductase, releasing calicheamicin (which breaks DNA double strands, thereby inducing apoptosis in tumor cells).
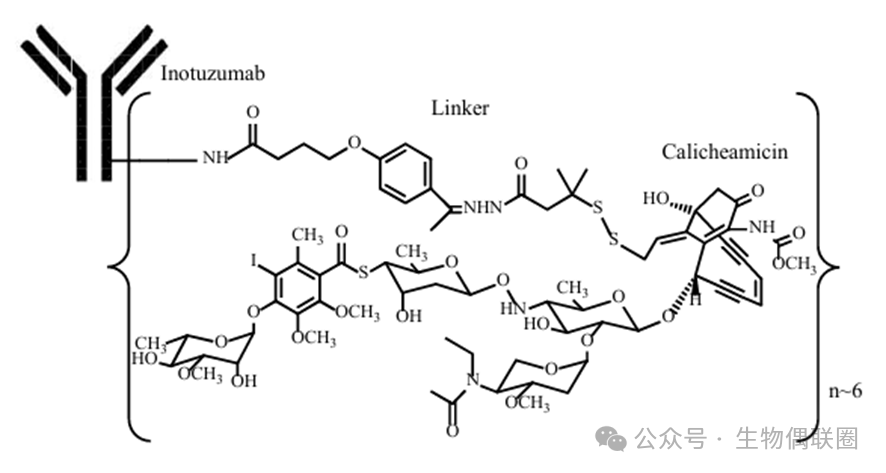
Figure 3 BesponsaStructural Diagram
Table 4 Summary of Besponsa Drug Information
|
Product Name |
Besponsa |
|
Generic Name |
Inotuzumab ozogamicin |
|
Manufacturer |
Pfizer, USA (Wyeth Pharma, a subsidiary of Pfizer) |
|
Indication |
Besponsa is indicated for adults with relapsed or refractory B-cell acute lymphoblastic leukemia |
|
Target |
CD22 |
|
Dosage Form |
Lyophilized Powder |
|
Specifications |
1mg/vial – 0.25mg/mL |
|
Buffer Type |
Buffer Salt: 18mM Ammonium Tris Excipient: 45mg/mL Sucrose, 5mg/mL Sodium Chloride Surfactant: 0.1mg/mL Polysorbate 80, pH 8.0 |
4.
Akalux Introduction
Akalux: A photoimmunotherapy ADC that needs to be used in conjunction with the BioBlade laser system during treatment.
Follow Xiaobai Learning Medicine
Linker-Payload: Photosensitive substance IRDye700DX (IR700), a water-soluble silicon phthalocyanine derivative, sensitive to red visible light.
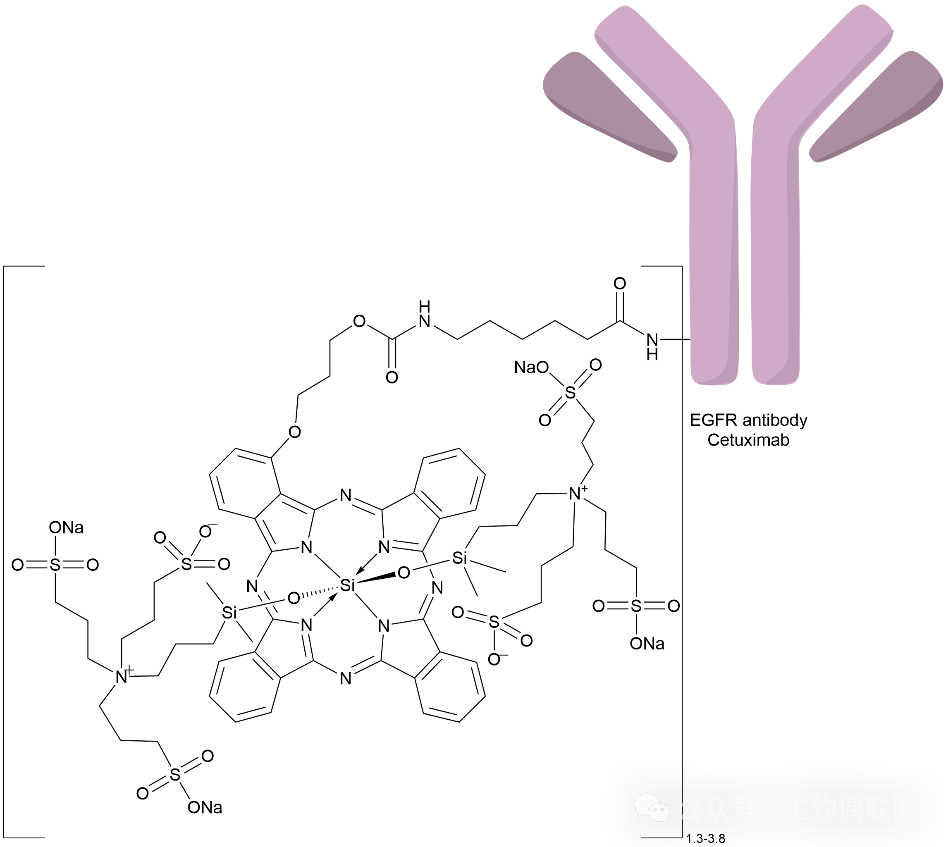
Figure 4 AkaluxStructural Diagram
Table 5 Summary of Akalux Drug Information
|
Product Name |
Akalux |
|
Generic Name |
Cetuximab sarotalocan |
|
Manufacturer |
Rakuten Medical, Japan |
|
Indication |
Colorectal cancer, head and neck cancer |
|
Target |
EGFR |
|
Dosage Form |
Lyophilized Powder |
|
Specifications |
250 mg/vial – 5mg/mL |
|
Buffer Type |
Buffer Salt: 10mM Phosphate Excipient: 90mg/mL Trehalose Surfactant: 0.2mg/mL Polysorbate 80, pH 7.1 |
5. Introduction to Elahere
Elahere: An antibody-drug conjugate targeting folate receptor (FRα) developed by ImmunoGen, consisting of the humanized anti-FRα monoclonal antibody M9346A of IgG1 subtype.
Linker: Sulfo-SPDB.Disulfide bond (S-S) linkageDM4, disulfide bond is reduced by cytoplasmic glutathione reductase, releasing calicheamicin
Payload: DM4, disulfide bond is reduced by cytoplasmic glutathione reductase, releasing active DM4, inhibiting microtubule polymerization and microtubule assembly, inducing effective anti-mitotic effects, leading to cell cycle arrest and apoptosis. Active metabolites may also diffuse to adjacent cells and induce further cell death.
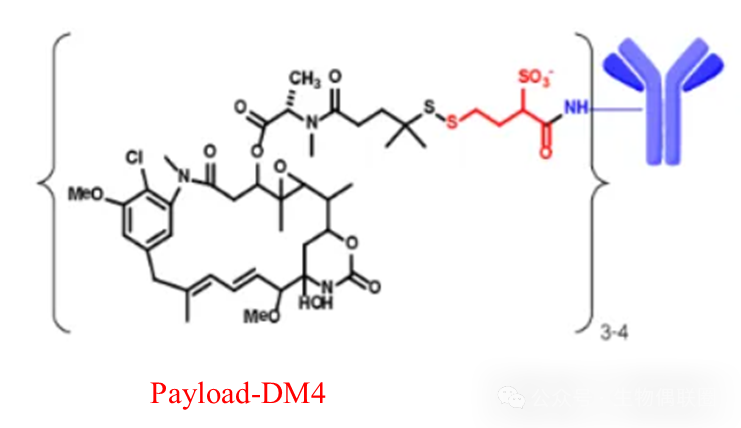
Figure 5 ElahereStructural Diagram
Table 6 Summary of Elahere Drug Information
|
Product Name |
Elahere 爱拉赫
|
|
Generic Name |
mirvetuximab / soravtansine-gynx |
|
Indication |
Epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer |
|
Target |
Folate Receptor α (FRα) |
|
Dosage Form |
Injection |
|
Specifications |
100mg/vial – 5mg/mL |
|
Buffer Type |
Buffer Salt: Acetic Acid 0.22mg/mL, 0.53mg/mL Sodium Acetate Excipient: 90mg/mL Sucrose Surfactant: 0.1mg/mL Polysorbate 20, pH 5.0 |
Follow Xiaobai Learning Medicine
Recommended Reading
From Follower to Leader: How Chinese Pharmaceutical Companies Rewrite the Global Landscape with ADC and Shielding Technology? |
Breaking Through Adversity! 2024 China Biopharmaceutical: Innovative Pharmaceutical Companies’ “Song of Ice and Fire” |
ADC: From “Precision Guidance” to “Technological Revolution”, Who Will Dominate the Trillion Market? |
Domestic ADC Sweeps Breast Cancer and Lung Cancer, Chinese Pharmaceutical Companies Capture 40% of R&D Pipeline |
Comprehensive Analysis of the Tumor Innovative Drug Industry: Technological Breakthroughs and Market Changes Rise and Future of Bispecific Antibody Drugs |
| ADC: From “Precision Guidance” to “Technological Revolution”, Who Will Dominate the Trillion Market? |
| Breakthroughs and Challenges in Domestic Biopharmaceuticals |
“Molecular-Level Collaborative Combat” of ADC Drugs: Optimization of Antibody-Toxin-Linker Triad Energy Field |
2025 China Innovative Drugs: Breakthroughs, Challenges, and Future |
Disclaimer:This public account does not constitute any investment opinions or advice.Reprint with source indicated:Xiaobai Learning Medicine Public Account。Some content is sourced from publicly available information on the internet, used for knowledge dissemination and learning, infringement will be deleted.Due to limited ability, if there are errors, please feel free to point them out.