
Among the 12 ADC drugs approved by the FDA, 5 utilize Seagen’s VC-MMAE linker toxin platform. As a pioneer in global ADC drug development, one might ask if Seagen has any regrets?They might respond that they missed the opportunity to develop ADC drugs targeting the classic targets HER2 and EGFR, and instead, they can only license a HER2 ADC drug from Rongchang Biopharma for a $200 million upfront payment and up to $2.6 billion in milestone payments.Dr. Hu Zhaohong, who previously served as a department director at Seagen, returned to China in 2014 to establish MeiYake Biopharma, developing the EGFR ADC drug MRG003 and the HER2 ADC drug MRG002. MeiYake Biopharma later became a subsidiary of Lepu Biopharma.On March 4, 2025, Lepu Biopharma announced that, based on the latest communication with regulatory authorities, it voluntarily withdrew the NDA application for the EGFR ADC drug MRG003 to supplement relevant materials and re-submitted the NDA on the same day.Further communication from the company revealed that the withdrawal of MRG003 was not due to clinical trial data issues, but rather due to errors in the application materials, which do not affect the market launch. The overall impact on the product’s timeline is approximately 1 to 3 months, with approval expected in 2026.The author does not wish to predict the future of MRG003, but it is essential to emphasize the historical significance behind its approval: it will be the world’s first truly approved EGFR ADC drug.As of now, according to publicly available information, Lepu Biopharma has established a pipeline of 7 ADC drugs, with 1 in NDA application stage, 2 in Phase III clinical trials, 3 in Phase I clinical trials, and 1 in preclinical stage, along with two overseas licensing agreements. In terms of development progress, one is the first globally, two are the second globally, and two are the first domestically. Overall, Lepu Biopharma, based on its acquisition of MeiYake Biopharma in July 2018, has entered the forefront of domestic ADC drug development, and this article provides a brief overview.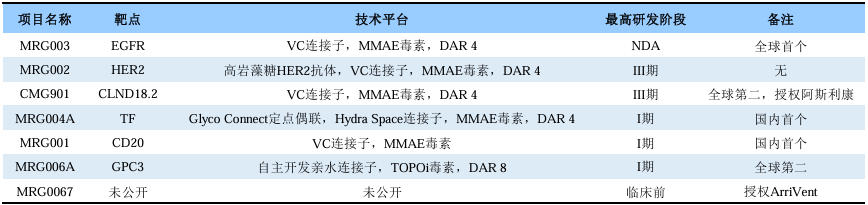 1
1 MRG003MRG003 is the first ADC drug developed by MeiYake Biopharma, with the antibody backbone derived from JMT101 (Becotatug), an EGFR antibody from Jinmant Biopharma, which has now been approved for market. JMT101 is an optimized version based on Amgen’s EGFR antibody Panitumumab, changing the antibody subtype from IgG2 to IgG1, with an affinity 7 times higher than the classic EGFR monoclonal antibody Cetuximab.In terms of ADC construction, it employs the classic VC-MMAE linker toxin platform, with a DAR value of 4. MRG003 first applied for IND in August 2016, was approved for clinical trials in August 2017, and the first patient was enrolled in May 2018.Structure design of MRG003
MRG003MRG003 is the first ADC drug developed by MeiYake Biopharma, with the antibody backbone derived from JMT101 (Becotatug), an EGFR antibody from Jinmant Biopharma, which has now been approved for market. JMT101 is an optimized version based on Amgen’s EGFR antibody Panitumumab, changing the antibody subtype from IgG2 to IgG1, with an affinity 7 times higher than the classic EGFR monoclonal antibody Cetuximab.In terms of ADC construction, it employs the classic VC-MMAE linker toxin platform, with a DAR value of 4. MRG003 first applied for IND in August 2016, was approved for clinical trials in August 2017, and the first patient was enrolled in May 2018.Structure design of MRG003
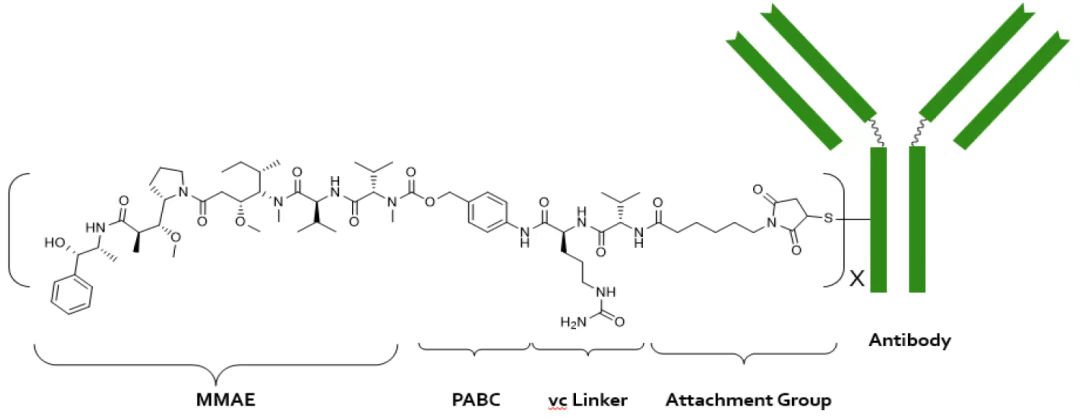 Source: Lepu Biopharma Official Website
Source: Lepu Biopharma Official Website
In July 2022, JAMA Oncology published the Phase I clinical data of MRG003. In the Ia dose escalation phase, among 22 patients, only 1 achieved a partial response at a dose of 2.5 mg/kg. In the Ib dose expansion phase, a dose of 2.5 mg/kg was used to treat 39 EGFR-positive patients, resulting in an overall response rate of 20.5%, a disease control rate of 51.3%, and a median PFS of 2.8 months.
In 10 patients with head and neck squamous cell carcinoma, the objective response rate was 40.0%, and the disease control rate was 100.0%, with a median PFS of 2.8 months. In the nasopharyngeal cancer subgroup, the objective response rate was 40.4%, the disease control rate was 89.0%, and the median PFS was 4.0 months. Based on these clinical results, MRG003 will prioritize further clinical trials in head and neck squamous cell carcinoma and nasopharyngeal cancer.
Phase I clinical data of MRG003
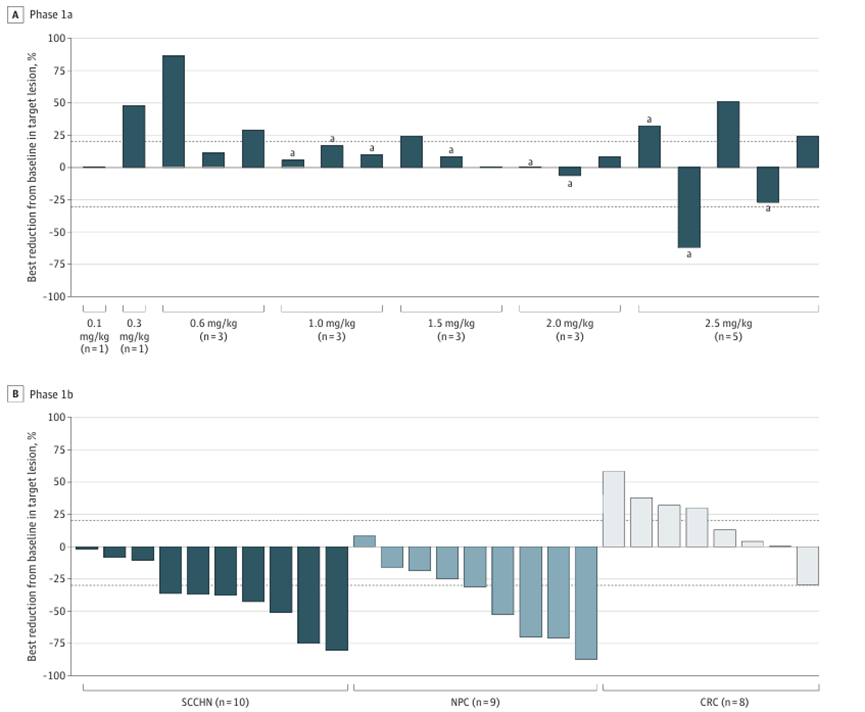 Source: JAMAOncol.2022;8(7):1042-1046
Source: JAMAOncol.2022;8(7):1042-1046
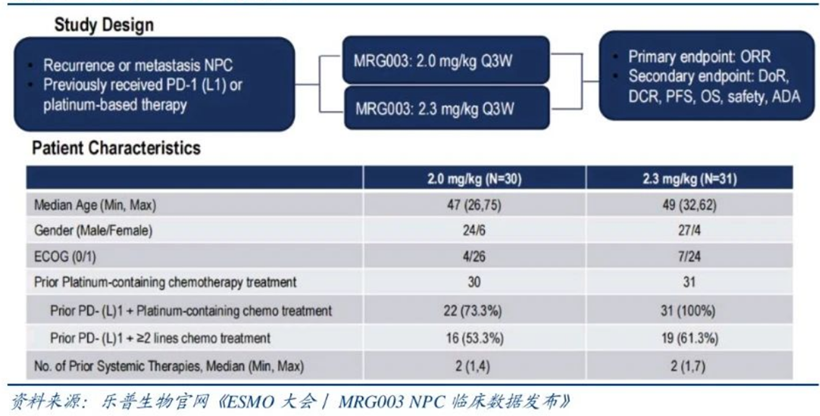
During the 2023 ESMO conference, Lepu Biopharma announced the latest data from the Phase IIa clinical trial of MRG003 for nasopharyngeal cancer, enrolling a total of 61 patients. As of March 15, 2023, efficacy assessments were conducted on 57 nasopharyngeal cancer patients who had previously received PD-(L)1 inhibitors and platinum-based chemotherapy.
Phase IIa clinical data of MRG003 for nasopharyngeal cancer
 Source: 2023 ESMO
Source: 2023 ESMO
The results showed an overall objective response rate of 47.4% and a disease control rate of 79.0%. In the 2.0 mg/kg dose group, among 28 patients, the objective response rate was 39.3%, and the disease control rate was 71.4%; while in the 2.3 mg/kg dose group, among 29 patients, the objective response rate was 55.2%, and the disease control rate was 86.2%. The median PFS in the 2.0 mg/kg dose group was 7.3 months, while the median PFS data in the 2.3 mg/kg dose group is not yet mature.
Phase IIa clinical data of MRG003 for nasopharyngeal cancer
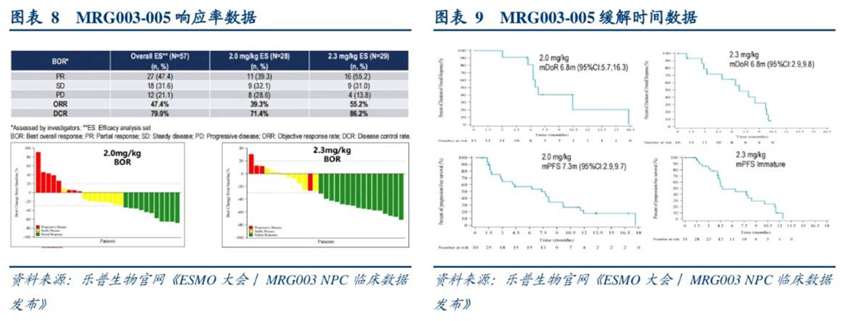 Source: 2023 ESMO
Source: 2023 ESMO
In terms of safety, the incidence of severe adverse effects was 11.5%, with 8 patients reducing the dose due to side effects, and 2 patients stopping treatment due to side effects, with no treatment-related deaths reported.
Phase IIa clinical data of MRG003 for nasopharyngeal cancer
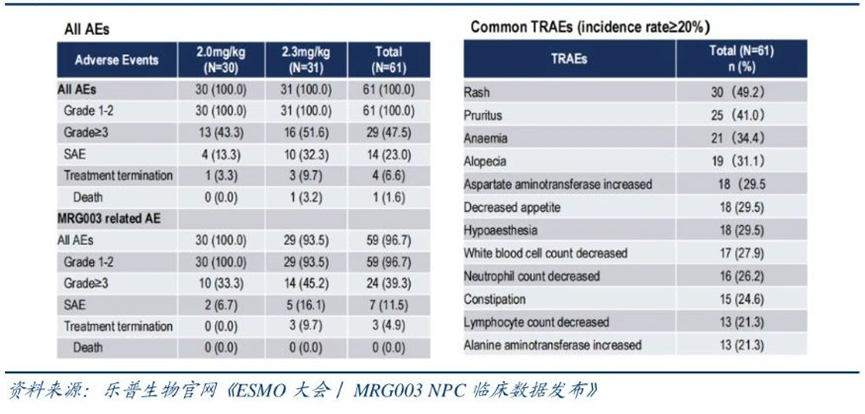 Source: 2023 ESMO
Source: 2023 ESMO
In August 2024, MRG003 received FDA breakthrough therapy designation for the treatment of recurrent or metastatic non-keratinizing nasopharyngeal carcinoma (NPC) in adult patients.In September 2024, the injection of Vebecotatug (MRG003) submitted an NMPA application for market approval, with the first indication being for patients with recurrent/metastatic nasopharyngeal carcinoma who have failed at least two lines of systemic chemotherapy and PD-1/PD-L1 inhibitors.As of now, MRG003 has submitted a market application for second-line and above treatment of advanced nasopharyngeal cancer, and is also conducting a Phase III clinical trial comparing it head-to-head with Cetuximab/methotrexate in advanced head and neck squamous cell carcinoma, along with multiple clinical trials for monotherapy or combination therapy.During the 2023 ESMO conference, MRG003 also announced Phase II clinical data for the treatment of head and neck squamous cell carcinoma. Among 67 patients, 35 received 2.0 mg/kg MRG003 treatment, and 32 received 2.3 mg/kg MRG003 treatment. The median line of treatment for patients was 2, with 95.6% having received platinum-based chemotherapy, 76.1% having received PD-1/L1 antibody treatment, and 47.8% having received Cetuximab treatment. In the 2.3 mg/kg dose group, among PD-1/L1 antibody resistant patients, the ORR was 43%, DCR was 86%, mPFS was 4.2 months, and mOS was 11.3 months.
Phase II clinical data of MRG003 for head and neck squamous cell carcinoma
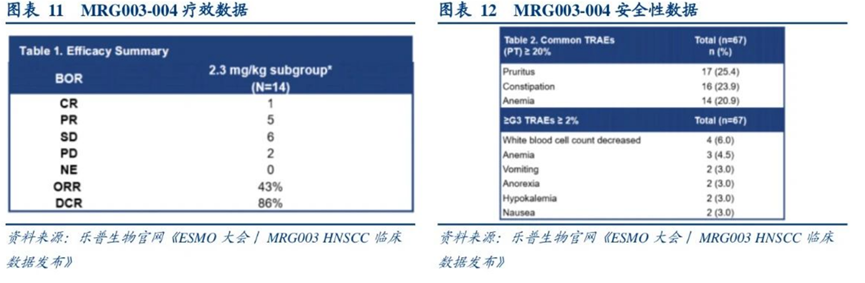 Source: 2023 ESMO
Source: 2023 ESMO
During the 2024 ASCO conference, Lepu Biopharma again announced the latest data from the I/II phase clinical trial of the combination of PD-1 antibody Putlilizumab and MRG003. As of January 30, 2024, the trial included 33 patients, with the I phase covering 9 nasopharyngeal cancer patients, 1 head and neck cancer patient, and 3 other solid tumor patients; the II phase included 14 nasopharyngeal cancer patients and 6 head and neck cancer patients. Among 27 patients evaluable for efficacy, the ORR was 63.0% (17/27), and the DCR was 88.9% (24/27).
Phase I/II clinical data of PD-1 + MRG003 combination therapy
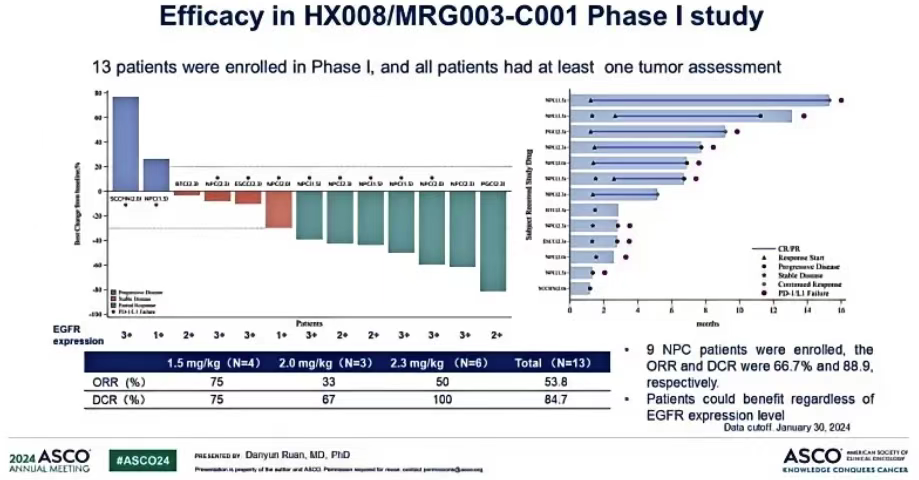 Source: 2024 ASCO
Source: 2024 ASCO
Based on preliminary clinical data, MRG003 is expected to provide new treatment options for nasopharyngeal cancer patients. Additionally, due to the widespread expression of EGFR in various solid tumors, it is anticipated to achieve new breakthroughs in indications such as head and neck squamous cell carcinoma and lung cancer, showing great promise.2 MRG002MRG002 is the second ADC drug developed by MeiYake. Its antibody component is an enhanced fucosylated antibody of Trastuzumab, which has a stronger affinity for HER2 protein and lower ADCC activity. The ADC construction also employs the classic VC-MMAE linker toxin platform, with a DAR value of 4.
MRG002MRG002 is the second ADC drug developed by MeiYake. Its antibody component is an enhanced fucosylated antibody of Trastuzumab, which has a stronger affinity for HER2 protein and lower ADCC activity. The ADC construction also employs the classic VC-MMAE linker toxin platform, with a DAR value of 4.
Product characteristics of MRG002
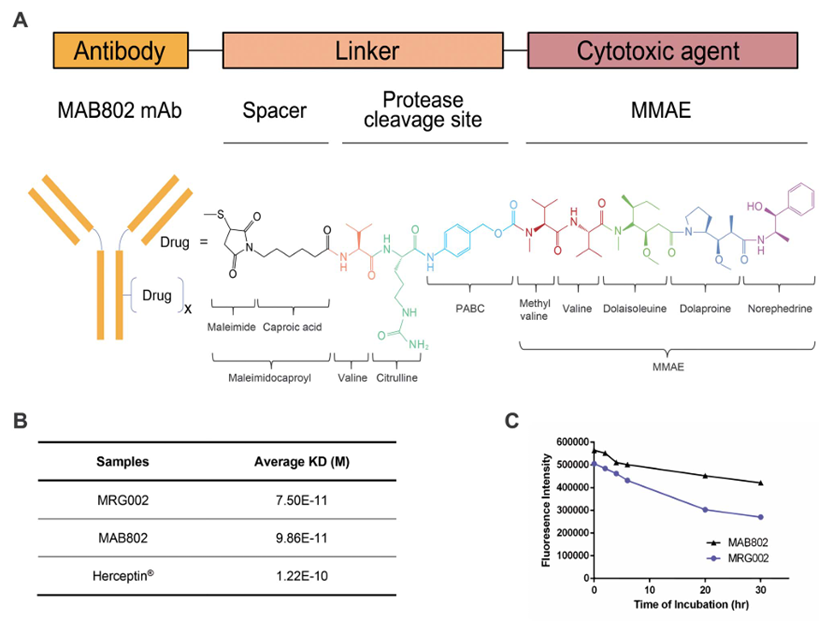 Source: Antib Ther. 2021 Aug 28;4(3):175-184
Source: Antib Ther. 2021 Aug 28;4(3):175-184
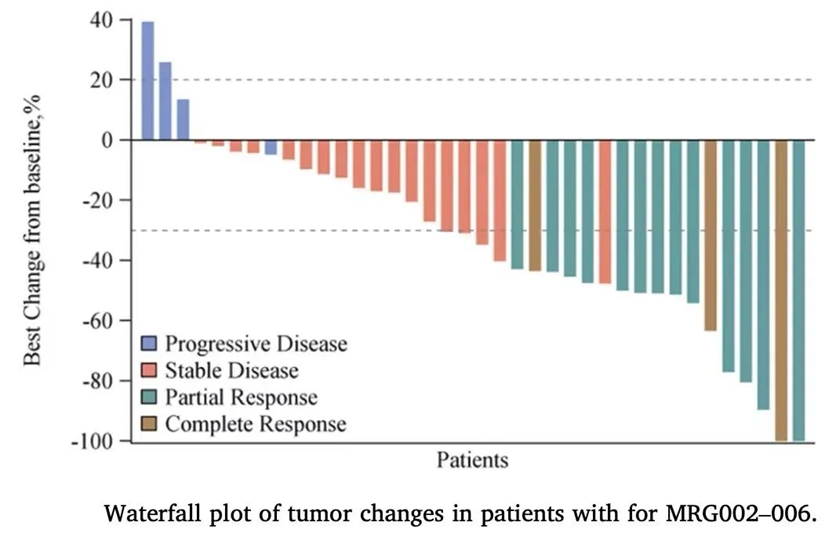
MRG002 first applied for clinical trials in November 2017 and received clinical approval in June 2018. During the CSCO conference in 2021, MRG002 announced the complete results of Phase I clinical trials for HER2-positive breast cancer and gastric cancer treatment.In 5 evaluable patients with HER2-positive gastric cancer who had previously received two lines of treatment and were resistant to Trastuzumab, the ORR was 60%, and the DCR was 80%. In 47 evaluable patients with HER2-positive breast cancer at doses above RP2D, the ORR reached 53%, with 61% ORR in 23 patients with liver metastases, 60% ORR in 5 patients with liver and brain metastases, and 68% ORR in 22 patients who were both HER2 and HR positive. ADC drugs are prone to interstitial pneumonia, ocular toxicity, or neurotoxicity. In this study, MRG002 did not report interstitial pneumonia or significant ocular toxicity.
Phase I clinical results of MRG002
 Source: 2021 CSCO
Source: 2021 CSCO
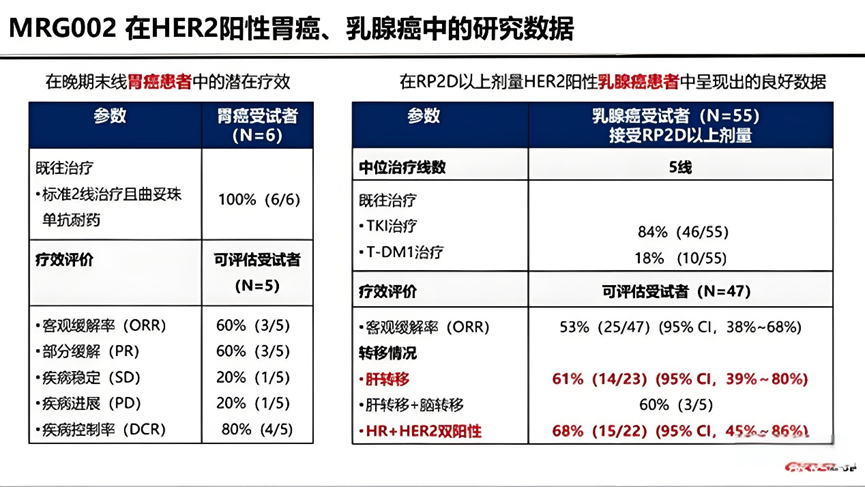
In July 2024, Lepu announced the Phase II clinical data of MRG002 in HER2-positive urothelial carcinoma. The ORR for patients treated with MRG002 was 53.4%, with a complete response (CR) of 6.9% and a disease control rate (DCR) of 83.7%. The median progression-free survival (PFS) and overall survival (OS) were 7.0 months and 14.9 months, respectively. Currently, MRG002 is initiating a Phase III clinical trial in HER2-positive locally advanced or metastatic urothelial carcinoma.
Phase II clinical data of MRG002 in HER2-positive urothelial carcinoma
 Source: Eur J Cancer. 2024 Jul:205:114096
Source: Eur J Cancer. 2024 Jul:205:114096
In the safety analysis of 43 patients, the most common treatment-related adverse events (AEs) were anemia (51.2%), alopecia (44.2%), and neutropenia (39.5%), most of which were grade 1 or 2. Among 34 patients treated with a dose of 2.2 mg/kg, the incidence of grade 3-4 treatment-related reactions (TRAE) was 44.1%, with only 2 patients (5.9%) terminating treatment due to adverse events.
In breast cancer, MRG002 has initiated a Phase III clinical trial comparing it head-to-head with Trastuzumab emtansine (T-DM1). During the 2024 SABCS conference, Lepu also updated the preliminary clinical results of MRG002 in HER2-positive metastatic breast cancer patients.
As of July 19, 2024, the median follow-up time was 14.8 months, with 5 patients (4.9%) having a treatment duration exceeding 2 years. The evaluated ORR was 60.8%, and the disease control rate (DCR) was 86.3%. The mPFS was 8.6 months, mDoR was 9.4 months, and mOS was 19.8 months.
Preliminary clinical results of MRG002 in HER2-positive metastatic breast cancer patients
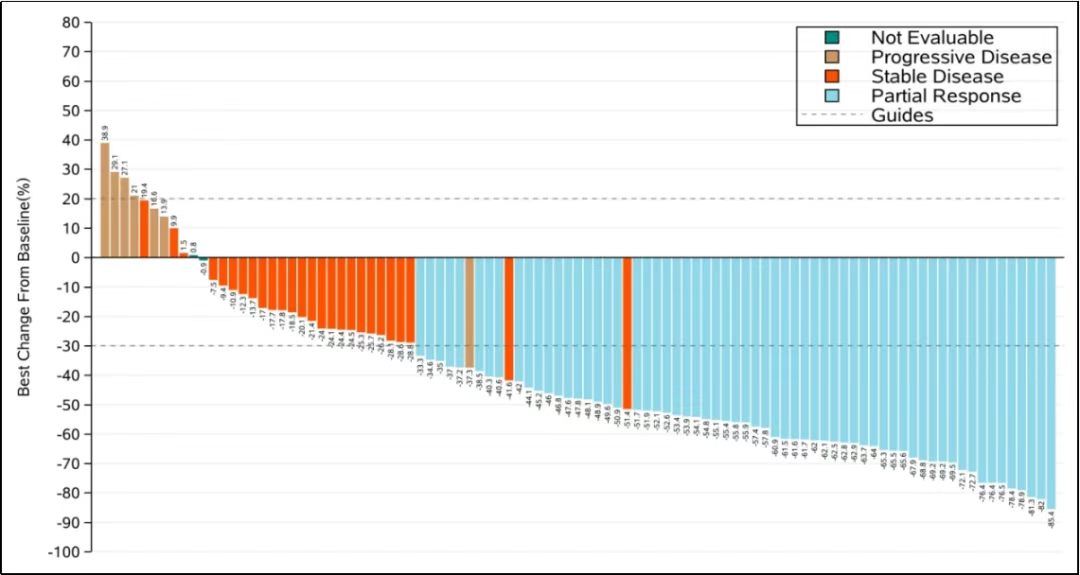 Source: 2024 SABCS
Source: 2024 SABCS
MRG002 has demonstrated significant anti-tumor activity in HER2-positive metastatic breast cancer patients who have failed Trastuzumab and anti-HER2 TKIs, with good patient tolerance and controllable safety. MRG002 is expected to become a new treatment option for this patient population. Additionally, Lepu has initiated a clinical exploration of PD-1 combined with MRG002 in advanced breast cancer.
Overall, MRG002 continues the development of Rongchang’s HER2 ADC, prioritizing Phase III clinical development in HER2-positive urothelial carcinoma. Although competition in breast cancer is fierce, MRG002’s head-to-head Phase III trial with T-DM1 is nearing completion, while differentiated exploratory development for liver metastatic patients is underway. Coupled with the support of PD-1 drugs in combination therapy, it has a strategic advantage for future group operations.
3 MRG001CD20 ADC drug MRG001 is the third ADC drug developed by MeiYake Biopharma, and it is also one of the only three CD20 ADC drugs globally. MRG001 is composed of a recombinant chimeric anti-CD20 monoclonal antibody MAB801 and a cytotoxic small molecule MMAE linked by a VC linker.MRG001 first applied for clinical trials in August 2018, and the first human dose escalation study has been completed, determining 1.8 mg/kg, administered once every 3 weeks, as its recommended dose for Phase II trials. At the 2023 ASH conference, the company reported the efficacy of MRG001 in R/R DLBCL patients who had progressed after at least two lines of treatment.This study included R/R DLBCL patients who had failed at least two lines of treatment (with one line requiring anti-CD20 treatment), with the primary endpoint being the ORR assessed by investigators. As of July 28, 2023, a total of 35 DLBCL patients were enrolled, with a median of 3 lines of prior treatment (ranging from 2 to 7 lines), including 3 patients who underwent autologous stem cell transplantation and 7 patients who received CAR-T treatment.The median follow-up time for the study was 6.8 months (range: 0.7 to 18.0 months), with 34 evaluable patients, resulting in an ORR of 38.2%. Among them, 6 patients achieved CR (17.6%); 7 patients achieved PR (20.6%); and 10 patients achieved SD (29.4%), resulting in a DCR of 67.7%. In patients who had not received CAR-T treatment, the ORR was 44.4% (12/27), and the DCR was 70.4%. The mDoR was 10.5 months, mPFS was 5.2 months, and mOS data is not yet mature, with a 12-month survival rate of 64.4%. The mPFS for patients who had not received CAR-T treatment was 6.3 months.Patients tolerated MRG001 well, with the most commonly reported treatment-related adverse events (TRAE) being leukopenia (68.6%), neutropenia (65.7%), anemia (45.7%), elevated AST (40.0%), and lymphopenia (31.4%). The most common grade 3/4 TRAEs were neutropenia (40.0%), leukopenia (17.1%), and lymphopenia (11.4%). The incidence of neurotoxicity for MRG001 was 5.7% (2/35), with all reactions being grade 1/2. Most patients’ TRAEs were manageable and resolved with symptomatic treatment, with only 2 patients terminating treatment due to TRAEs, and no patients died due to TRAEs.4
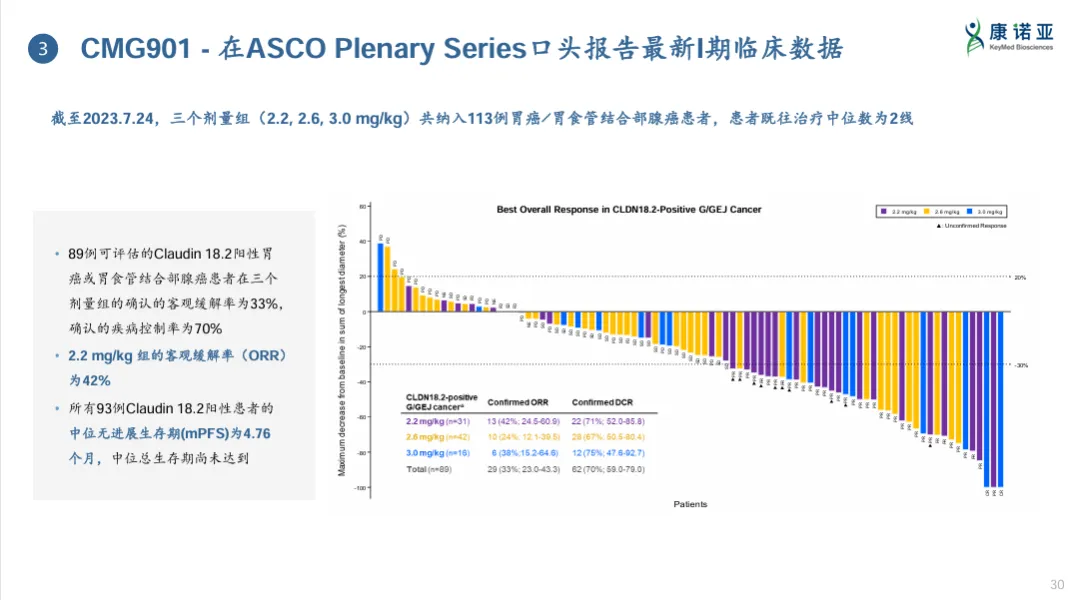
MRG001CD20 ADC drug MRG001 is the third ADC drug developed by MeiYake Biopharma, and it is also one of the only three CD20 ADC drugs globally. MRG001 is composed of a recombinant chimeric anti-CD20 monoclonal antibody MAB801 and a cytotoxic small molecule MMAE linked by a VC linker.MRG001 first applied for clinical trials in August 2018, and the first human dose escalation study has been completed, determining 1.8 mg/kg, administered once every 3 weeks, as its recommended dose for Phase II trials. At the 2023 ASH conference, the company reported the efficacy of MRG001 in R/R DLBCL patients who had progressed after at least two lines of treatment.This study included R/R DLBCL patients who had failed at least two lines of treatment (with one line requiring anti-CD20 treatment), with the primary endpoint being the ORR assessed by investigators. As of July 28, 2023, a total of 35 DLBCL patients were enrolled, with a median of 3 lines of prior treatment (ranging from 2 to 7 lines), including 3 patients who underwent autologous stem cell transplantation and 7 patients who received CAR-T treatment.The median follow-up time for the study was 6.8 months (range: 0.7 to 18.0 months), with 34 evaluable patients, resulting in an ORR of 38.2%. Among them, 6 patients achieved CR (17.6%); 7 patients achieved PR (20.6%); and 10 patients achieved SD (29.4%), resulting in a DCR of 67.7%. In patients who had not received CAR-T treatment, the ORR was 44.4% (12/27), and the DCR was 70.4%. The mDoR was 10.5 months, mPFS was 5.2 months, and mOS data is not yet mature, with a 12-month survival rate of 64.4%. The mPFS for patients who had not received CAR-T treatment was 6.3 months.Patients tolerated MRG001 well, with the most commonly reported treatment-related adverse events (TRAE) being leukopenia (68.6%), neutropenia (65.7%), anemia (45.7%), elevated AST (40.0%), and lymphopenia (31.4%). The most common grade 3/4 TRAEs were neutropenia (40.0%), leukopenia (17.1%), and lymphopenia (11.4%). The incidence of neurotoxicity for MRG001 was 5.7% (2/35), with all reactions being grade 1/2. Most patients’ TRAEs were manageable and resolved with symptomatic treatment, with only 2 patients terminating treatment due to TRAEs, and no patients died due to TRAEs.4 Collaborative Win-WinWith the advancement of three ADC drugs in clinical trials, MeiYake can be considered part of the first group of domestic ADC drug developers. Based on this solid foundation, in July 2018, Lepu Biopharma acquired MeiYake Biopharma and began accelerating the development of ADC drugs, upgrading the R&D platform.First, in collaboration with Kanyua Biopharma, they developed the CLDN18.2 ADC drug CMG901, which is also the second ADC drug globally to enter Phase III clinical trials. The antibody for CMG901 is developed by Kanyua, and the ADC construction employs the classic VC-MMAE linker toxin platform, with a DAR value of 4.CMG901 first applied for IND in August 2020. In March 2023, Kanyua/Lepu Biopharma reached a cooperation agreement with AstraZeneca worth over $1.1 billion, including an upfront payment of $63 million, making it the first CLDN18.2 ADC drug to go overseas.In the clinical trial of CMG901, the expression of CLDN18.2 in enrolled patients was strictly controlled to be over 20%. Data presented at the 2024 ASCO conference showed that among 89 evaluable gastric cancer/gastroesophageal junction adenocarcinoma subjects in the 2.2 mg/kg, 2.6 mg/kg, and 3.0 mg/kg dose groups, the confirmed overall ORR was 35%, and the DCR was 70%, with an ORR of 42% in the 2.2 mg/kg dose group. However, it is noteworthy that the response rates and PFS in the three dose groups did not show a dose-dependent relationship.
Collaborative Win-WinWith the advancement of three ADC drugs in clinical trials, MeiYake can be considered part of the first group of domestic ADC drug developers. Based on this solid foundation, in July 2018, Lepu Biopharma acquired MeiYake Biopharma and began accelerating the development of ADC drugs, upgrading the R&D platform.First, in collaboration with Kanyua Biopharma, they developed the CLDN18.2 ADC drug CMG901, which is also the second ADC drug globally to enter Phase III clinical trials. The antibody for CMG901 is developed by Kanyua, and the ADC construction employs the classic VC-MMAE linker toxin platform, with a DAR value of 4.CMG901 first applied for IND in August 2020. In March 2023, Kanyua/Lepu Biopharma reached a cooperation agreement with AstraZeneca worth over $1.1 billion, including an upfront payment of $63 million, making it the first CLDN18.2 ADC drug to go overseas.In the clinical trial of CMG901, the expression of CLDN18.2 in enrolled patients was strictly controlled to be over 20%. Data presented at the 2024 ASCO conference showed that among 89 evaluable gastric cancer/gastroesophageal junction adenocarcinoma subjects in the 2.2 mg/kg, 2.6 mg/kg, and 3.0 mg/kg dose groups, the confirmed overall ORR was 35%, and the DCR was 70%, with an ORR of 42% in the 2.2 mg/kg dose group. However, it is noteworthy that the response rates and PFS in the three dose groups did not show a dose-dependent relationship.
Phase I clinical results of CMG901
 Source: Kanyua Official Website
Source: Kanyua Official Website
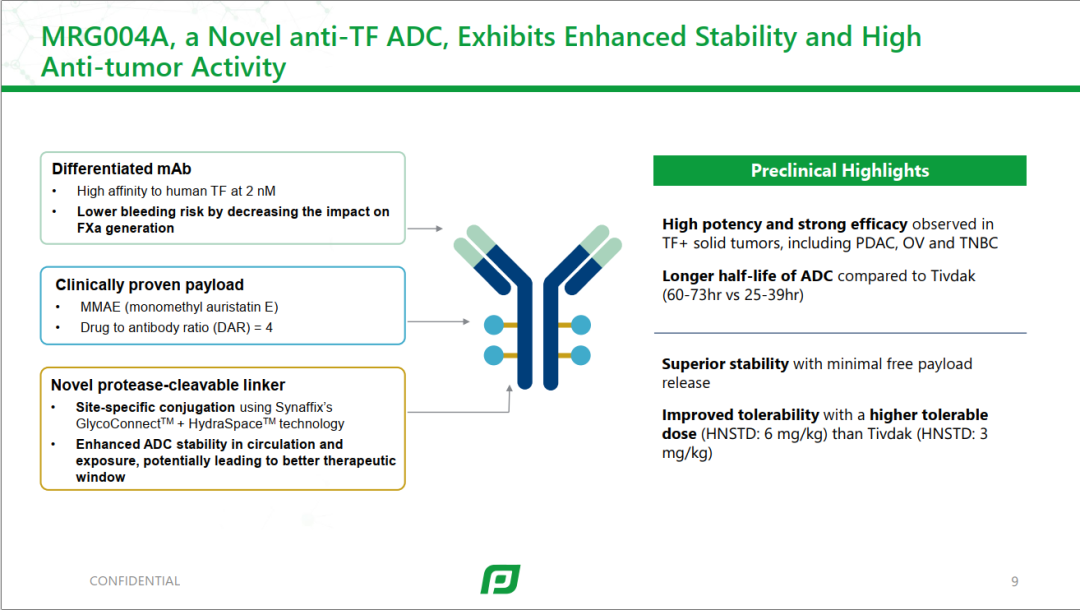
Currently, CMG901 (AZD0901) is undergoing international, multi-center Phase III research globally (NCT06346392), exploring the efficacy and safety of CMG901 (AZD0901) monotherapy compared to investigator-selected standard treatment in patients with advanced solid tumors expressing CLDN18.2. It is expected to receive simultaneous approval in China and Western countries in the future, bringing hope to patients with advanced gastric cancer worldwide.5 Platform UpgradeIn addition to product collaborations and licensing, Lepu has also upgraded the ADC drug platform.MRG004A is an ADC drug targeting a differentiated target, using innovative conjugation technology for tissue factor (TF), linked through Glyco Connect™ site-specific conjugation and Hydra Space™ polar spacer technology, connecting TF-targeting monoclonal antibodies with the highly effective anti-microtubule agent MMAE. It is also the first TF ADC drug to enter clinical trials in China.
Platform UpgradeIn addition to product collaborations and licensing, Lepu has also upgraded the ADC drug platform.MRG004A is an ADC drug targeting a differentiated target, using innovative conjugation technology for tissue factor (TF), linked through Glyco Connect™ site-specific conjugation and Hydra Space™ polar spacer technology, connecting TF-targeting monoclonal antibodies with the highly effective anti-microtubule agent MMAE. It is also the first TF ADC drug to enter clinical trials in China.
Product structure of MRG004A
 Source: Lepu Biopharma Communication Meeting
Source: Lepu Biopharma Communication Meeting
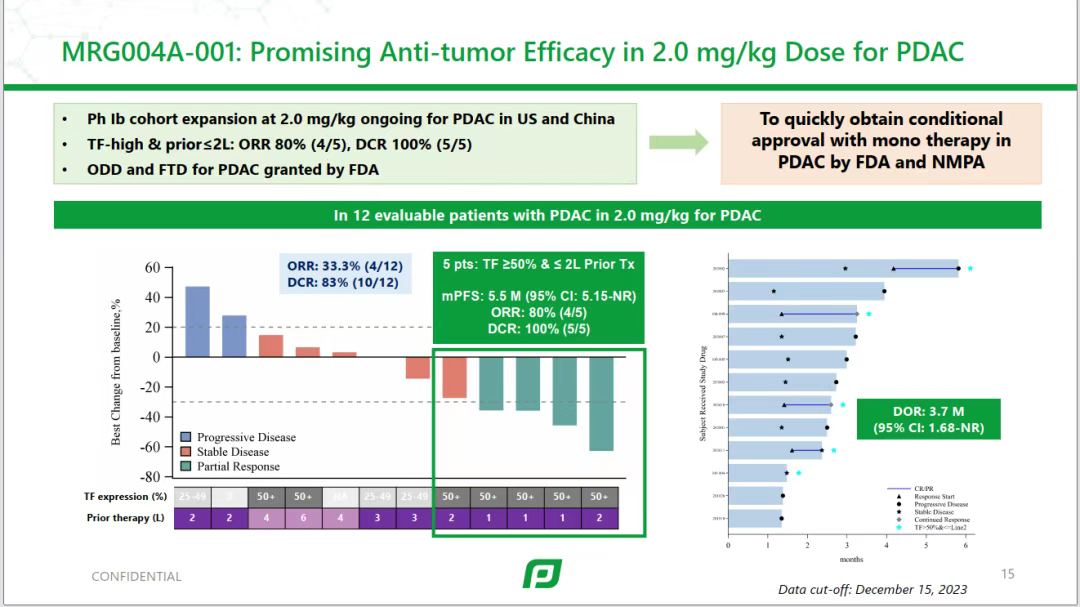
MRG004A has obtained IND approvals in both China and the United States, and has been granted orphan drug designation and fast track designation by the FDA for the treatment of pancreatic cancer. It is currently undergoing Phase I clinical research in the United States and China, and has shown anti-tumor activity signals in indications such as pancreatic cancer, triple-negative breast cancer, and colorectal cancer.During the 2024 ASCO conference, MRG004A first announced Phase I clinical data. In the 2.0 mg/kg dose group, among 12 evaluable pancreatic cancer patients (with a median of 3 lines of prior treatment), there were 4 cases of partial response (PR) and 6 cases of stable disease (SD). The objective response rate (ORR) was 33.3% (4/12), and the disease control rate (DCR) was 83.3% (10/12). Among 5 pancreatic cancer patients with TF expression rate ≥50% and intensity of 3+, after receiving 2 mg/kg treatment, 4 achieved PR, and 1 achieved SD.
Phase I clinical results of MRG004A
 Source: Lepu Biopharma Communication Meeting
Source: Lepu Biopharma Communication Meeting
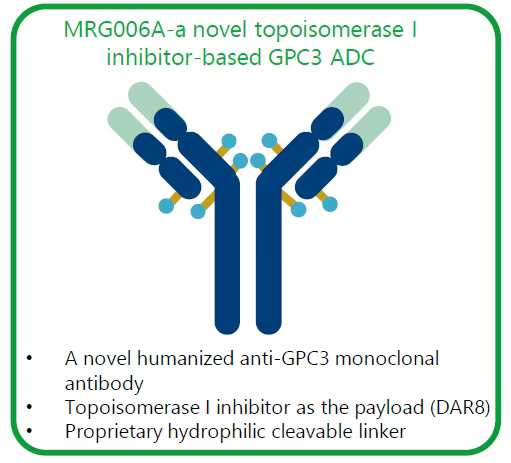
At the same time, MRG004A has also shown efficacy in other cancer types. In 4 patients with triple-negative breast cancer (TNBC) who had received multiple lines of treatment, the ORR and DCR were 25% (1/4) and 50% (2/4), respectively. In 2 patients with cervical cancer who had previously received 4 lines of treatment, 1 achieved PR, and 1 achieved SD.In terms of safety, common treatment-related adverse events (TRAE) included conjunctivitis (27%), anemia (17%), and hypoalbuminemia (13%), with 5 patients (7.9%, 5/63) experiencing serious adverse events. One TNBC patient receiving 1.8 mg/kg treatment experienced grade 3 Stevens-Johnson syndrome, which was a dose-limiting toxicity (DLT) and has since recovered. No other DLTs were observed, and dose expansion and mature results evaluation are ongoing.TF is a mature target, and MRG004A is optimized based on Pfizer’s marketed products through site-specific integration, providing both certainty and innovative exploration space. Particularly, it has achieved preliminary positive data in pancreatic cancer, bringing breakthrough highlights for the product’s future.In addition to exploring site-specific conjugation technology, Lepu is also laying out plans for novel toxins.During the 2024 AACR, Lepu announced the first ADC drug with a topoisomerase inhibitor toxin, MRG006A, which uses a humanized GPC3 antibody, a topoisomerase inhibitor toxin, and a self-developed water-soluble cleavable linker, with a DAR value of 8.
Product structure of MRG006A
 Source: 2024 AACR
Source: 2024 AACR
MRG006A has shown good anti-tumor effects in various CDX and PDX tumor models in preclinical studies. MRG006A first applied for clinical trials in April 2024, becoming the second GPC3 ADC drug to enter clinical stages globally, with promising clinical prospects.
In addition, on January 22, 2025, Lepu Biopharma announced an exclusive licensing agreement with ArriVent Biopharma, Inc., with a total upfront payment and recent milestone payments of $47 million, and up to $1.16 billion in milestone payments, granting the latter rights to the innovative ADC drug MRG007 outside of the Greater China region.It is reported that MRG007 has shown good anti-tumor activity in preclinical models of gastrointestinal cancers, demonstrating a high therapeutic index in IND supportive studies. The first IND application is planned to be submitted in the first half of 2025, with preliminary clinical development focusing on colorectal cancer, pancreatic cancer, and other gastrointestinal malignancies.Lepu Biopharma is known for its resource integration. Through the acquisition of MeiYake, it has a rich ADC pipeline, with multiple products entering the registration clinical stage, and many products’ development progress is at the forefront both internationally and domestically. Looking back at the development history of MRG003, it has accompanied the acceleration of innovative drug development in China and the reform of drug regulatory systems, hoping for a rapid approval after unexpected delays. Meanwhile, Lepu Biopharma, based on the approved PD-1 monoclonal antibody Putlilizumab, is already showing promise in combination with ADC drugs, expected to fully realize pipeline value in the IO+ADC wave. The story of Lepu Biopharma’s ADC drugs is far from over.*Disclaimer: This article is solely for introducing research progress in the field of medicine and diseases, summarizing research overviews, or sharing related medical information. It does not and will not recommend treatment or diagnostic plans, nor does it constitute any investment advice.If there are any omissions in the content, please feel free to communicate!
Amgen’s mRNA shingles vaccine has received FDA approval for clinical trials, with a leading player in the trillion-dollar market emerging!
$2 billion! The first metabolic triple agonist from China goes overseas!
Revenue surged by 461%! What did “AI + innovative drugs” first stock Yunding Xinyao do right?


