Updates on the first human study data from Phase I/II show that B7-H3 targeted ADCs continue to demonstrate therapeutic potential.
In recent years, antibody-drug conjugates (ADCs) have emerged as a new class of drugs that bring new “hope” to the treatment of advanced cancer patients due to their unique mechanisms of action. The B7 family consists of a group of immune regulatory ligands that regulate T cell activation and differentiation, expressed in acquired immune cells, innate immune cells, and various cancer tissues. Among the ten known members of the B7 family are B7-H1 (PD-L1) and B7-H3. B7-H3 has gained attention due to its high expression in various cancers and its association with poor prognosis. Ifinatamab Deruxtecan (I-DXd; DS-7300) is a B7-H3 targeted ADC drug with a topoisomerase I inhibitor payload (DXd). The latest results from its Phase I/II study presented at the 2023 European Society for Medical Oncology (ESMO) (abstract number: 690P) [1] show that in heavily pre-treated patients, the objective response rate (ORR) for small cell lung cancer (SCLC) patients was 52.4%, with a median progression-free survival (mPFS) of 5.6 months and a median overall survival (mOS) of 12.2 months; for squamous non-small cell lung cancer (sqNSCLC) patients, the ORR was 30.8%, mPFS was not reached (NR), and mOS was NR; and the overall safety and tolerability were good.
Background: Immunotherapy improves first-line survival benefits for SCLC and sqNSCLC patients, but the efficacy of subsequent treatments remains unsatisfactory.
SCLC accounts for approximately 13%-15% of lung cancer cases, with about 250,000 new cases reported globally each year, leading to at least 200,000 deaths. SCLC is characterized by rapid proliferation, early dissemination, and poor prognosis, with a five-year survival rate of less than 7% [2]. For decades, clinical treatment regimens for SCLC have changed little, with the etoposide and platinum (EP) regimen being the standard treatment. It was not until recent years that the combination of anti-PD-L1 immunotherapy with EP chemotherapy further improved survival rates for SCLC patients. However, SCLC patients inevitably develop rapid resistance, and existing treatment options beyond the first line are very limited and have poor efficacy. With a deeper understanding of the mechanisms of SCLC and the rapid development of molecular targeted therapies, several targeted drugs have been explored in the treatment of SCLC, but none have provided satisfactory therapeutic benefits [2].
- A Phase II clinical study (NCT01642251) showed that the PARP inhibitor Veliparib combined with the EP regimen extended OS by 1.4 months in SCLC patients, but the results were not statistically significant, and predictive biomarkers are still needed to enhance the clinical efficacy of PARP inhibitors[3].
- DLL3 is a potential therapeutic target for SCLC, but its targeted drug Rovalpituzumab did not show advantages in efficacy and safety in the Phase II TRINITY study[4], and the Phase III TAHOE study was temporarily terminated due to poor OS and high toxicity[5].Preliminary data on the DLL3/CD3 bispecific antibody tarlatamab showed good safety, with a confirmed ORR of 23% in heavily pre-treated SCLC patients, and a Phase III study is ongoing[6].
- BCL-2 antagonists are also potential treatment options, but the targeted drug Navitoclax showed extremely limited activity in single-agent trials for advanced and relapsed SCLC, with 41% of patients experiencing grade 3-4 thrombocytopenia[7].
- Additionally, studies have found that AURK combined with chemotherapy can inhibit tumor proliferation and growth, and the AURK inhibitor Alisertib combined with paclitaxel can improve PFS and OS[8].Some preclinical studies suggest that c-kit receptor, EGFR, insulin-like growth factor receptor, and cMET inhibitors may be effective for SCLC, but unfortunately, subsequent clinical trials have failed to demonstrate that these drugs are superior to existing treatment standards.
NSCLC accounts for approximately 80-85% of all lung cancers, with about 20-30% being sqNSCLC[9].Most sqNSCLC patients are diagnosed at an advanced stage, with limited systemic treatment options and high disease-related mortality rates.In recent years, tumor immunotherapy has progressed rapidly in lung cancer, with PD-1 inhibitors combined with carboplatin + albumin-bound paclitaxel being consistently recommended by authoritative guidelines both domestically and internationally, becoming one of the mainstream treatment options for first and second lines.However, the efficacy of second-line immunotherapy remains low for unselected sqNSCLC patients.In the CheckMate 017 trial, the efficacy rate of nivolumab treatment in unselected sqNSCLC patients in the second line was approximately 20%[10].Although targeted therapy exploration for sqNSCLC has been actively conducted, the efficacy remains unsatisfactory.Studies show that the frequency of EGFR mutations in the Chinese sqNSCLC population is approximately 4.3%, ALK mutations are 2.0%, and KRAS mutations are 1.7%[11].Due to the low frequency of these high-frequency driver genes found in other types of lung cancer in sqNSCLC, the efficacy of second-line targeted therapy for patients remains poor.The LUX-Lung 8 study results showed that in unselected sqNSCLC patients who had previously received first-line chemotherapy, the mPFS for second-line treatment with afatinib and erlotinib was only 2.6 months and 1.9 months, respectively, with mOS of 7.9 months and 6.8 months[12].Furthermore, studies have found that FGFR1 amplification, PI3K pathway mutations, and G1/S checkpoint mutations (such as CDKN2A mutations) in sqNSCLC are potential druggable targets, but previous early clinical studies showed that the ORR for sqNSCLC patients receiving corresponding targeted therapies was only 4%-11.5%, with mPFS of only 1.8-2.9 months[13].Overall, treatment options and efficacy for SCLC and sqNSCLC are very limited, especially for second-line and subsequent treatments, highlighting a significant unmet clinical need for exploring more effective treatment options to improve patient survival benefits.
Study Design
The Phase I/II first human study of I-DXd included patients with advanced solid tumors without selection for B7-H3 expression, receiving 4.8-16 mg/kg I-DXd Q3W treatment. Efficacy was assessed in patients who had two baseline scans or discontinued treatment for any reason, and retrospective evaluation of patients’ B7-H3 expression levels was conducted. The key primary endpoints were ORR based on RECIST v1.1 assessment, duration of response (DOR), disease control rate (DCR), PFS, OS, and safety, with key secondary endpoints including pharmacokinetic characteristics and immunogenicity.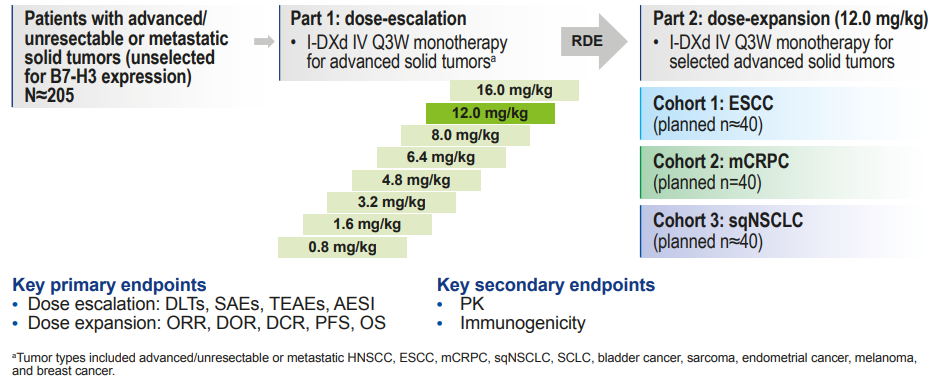 Figure 1. Study design of the Phase I/II first human trial of I-DXd
Figure 1. Study design of the Phase I/II first human trial of I-DXd
Study Results
▌Patient Baseline Characteristics
As of January 31, 2023, 8 patients were still receiving treatment. 14 patients were excluded from the ORR analysis due to a lack of evaluable baseline target lesions. Among SCLC, sqNSCLC, ESCC, and CRPC patients, the median number of prior treatments was 2 (1-9), 3 (1-12), 4 (1-7), and 6 (1-11), respectively. Most patients had high or moderate expression of B7-H3.
▌Efficacy Results
-
A total of 21 SCLC patients, with a median number of prior treatment lines of 2 (1-7), had an ORR of 52.4%, mDOR of 5.9 months, mPFS of 5.6 months, and mOS of 12.2 months.
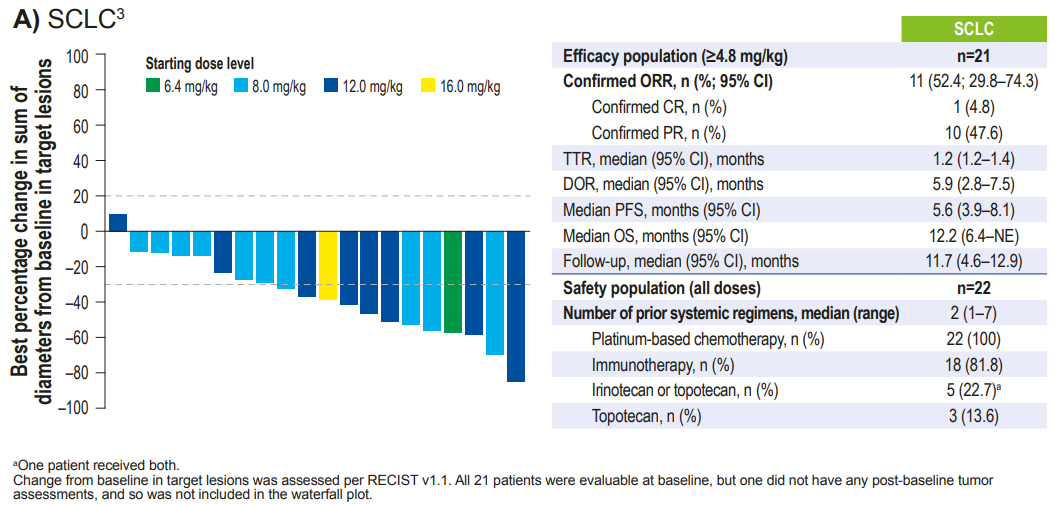 Figure 2. Latest follow-up data from the Phase I/II first human trial of I-DXd – SCLC cohort
Figure 2. Latest follow-up data from the Phase I/II first human trial of I-DXd – SCLC cohort
- A total of 13 sqNSCLC patients, with a median number of prior treatment lines of 3 (1-12), had an ORR of 30.8%, mDOR of 4.1 months, and mPFS and mOS were NR.
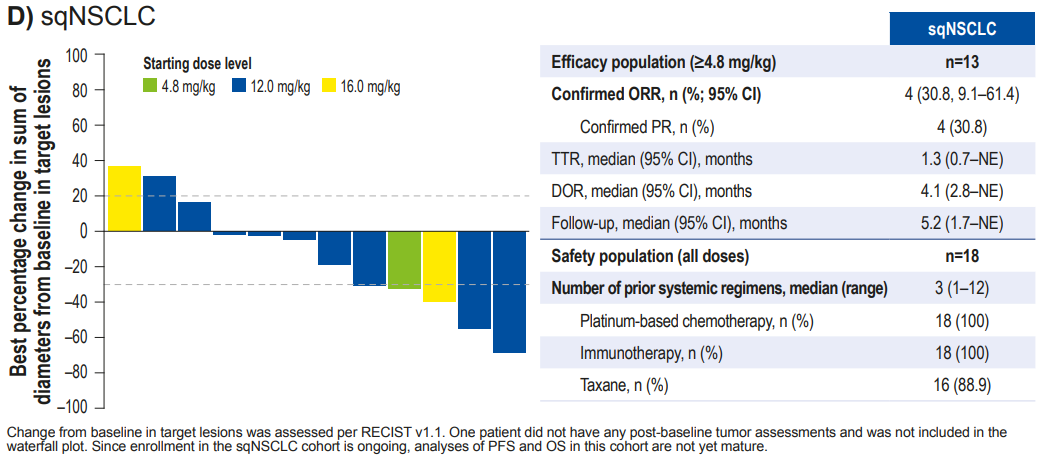 Figure 3. Latest follow-up data from the Phase I/II first human trial of I-DXd – squamous NSCLC cohort
Figure 3. Latest follow-up data from the Phase I/II first human trial of I-DXd – squamous NSCLC cohort
- Additionally, I-DXd has also shown excellent efficacy in other solid tumors.
- A total of 28 ESCC patients, with a median number of prior treatment lines of 4 (1-7), had an ORR of 21.4%, mDOR of 3.5 months, mPFS of 2.8 months, and mOS of 7.0 months.
- A total of 73 mCRPC patients, with a median number of prior treatment lines of 6 (1-11), had an ORR of 25.4%, mDOR of 6.4 months, mPFS of 5.3 months, and mOS of 13.0 months.
▌
Safety ResultsSimilar to previous data, no new adverse events were observed. The incidence of treatment-emergent adverse events (TEAEs) of grade ≥3 was 43.7%, with only 8.0% of patients discontinuing treatment due to TEAEs. The most common grade ≥3 TEAEs included anemia (19.0%), neutropenia (4.0%), nausea (3.4%), and lymphopenia (3.4%). Overall safety and tolerability were good.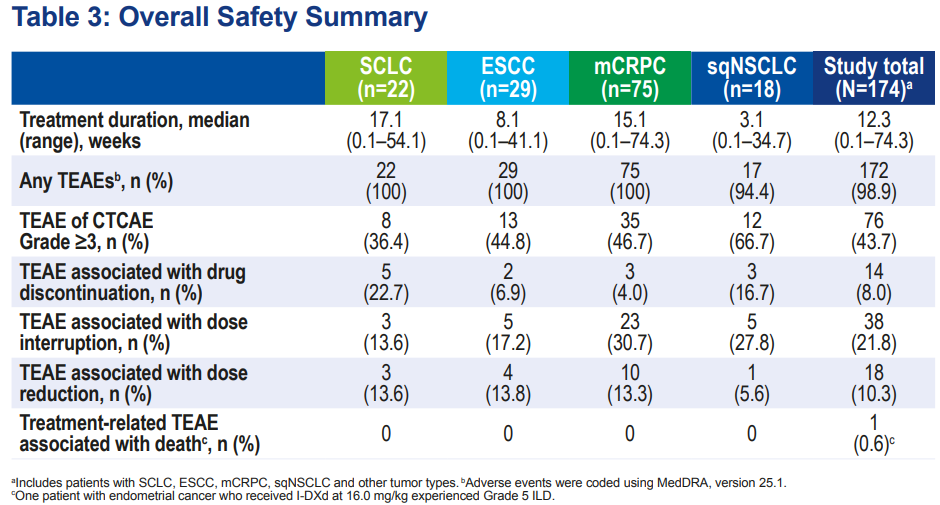 Figure 4. Latest follow-up data from the Phase I/II first human trial of I-DXd – summary of safety data
Figure 4. Latest follow-up data from the Phase I/II first human trial of I-DXd – summary of safety data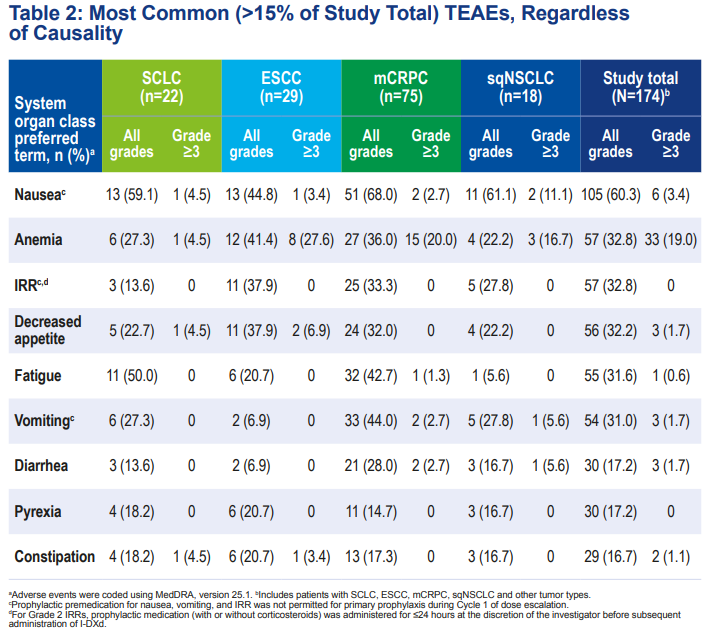 Figure 5. Latest follow-up data from the Phase I/II first human trial of I-DXd – most common adverse event data
Figure 5. Latest follow-up data from the Phase I/II first human trial of I-DXd – most common adverse event data
Conclusion
The development of molecular targeted and immunotherapy has significantly improved the survival of cancer patients, but the efficacy in tumors such as SCLC and sqNSCLC remains unsatisfactory, especially for second-line and subsequent treatments, which require further exploration. ADC drugs have shown excellent efficacy in the field of solid tumors, bringing new treatment options and hope to cancer patients. The latest follow-up data from the 2023 ESMO shows that I-DXd achieved an ORR of 52% in heavily pre-treated SCLC patients, with mPFS of 5.9 months and mOS of 9.9 months, promising to further improve the poor prognosis and survival of SCLC patients. Additionally, sqNSCLC patients lack potential targetable genomic alterations, while heavily pre-treated sqNSCLC patients can benefit from I-DXd treatment, regardless of B7-H3 expression levels. I-DXd may break through the current treatment dilemma, providing new treatment options for sqNSCLC patients. Furthermore, I-DXd has also shown promising anti-tumor activity in ESCC and CRPC, achieving encouraging DOR and OS, consistent with previous research results. We look forward to further disclosure of research data and proactive layouts in these treatment areas, providing more evidence for the efficacy and safety of I-DXd in solid tumors.
References:
[1] Patel M.R, Doi T, Koyama T, et al. Ifinatamab deruxtecan (I-DXd; DS-7300) in patients with advanced solid tumors: Updated clinical and biomarker results from a phase I/II study. 2023 ESMO 690P.
[2] Megyesfalvi Z, Gay CM, Popper H, et al. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin. 2023;10.3322/caac.21785.
[3] Owonikoko TK, Dahlberg SE, Sica GL, et al. Randomized phase II trial of cisplatin and etoposide in combination with veliparib or placebo for extensive‐stage small‐cell lung cancer: ECOG‐ACRIN 2511 study. J Clin Oncol. 2019;37(3):222‐229.
[4] Morgensztern D, Besse B, Greillier L, et al. Efficacy and safety of rovalpituzumab tesirine in third‐line and beyond patients with DLL3‐expressing, relapsed/refractory small‐cell lung cancer: results from the phase II TRINITY study. Clin Cancer Res. 2019;25(23):6958‐6966.
[5] Owen DH, Giffin MJ, Bailis JM, Smit MAD, Carbone DP, He K. DLL3: an emerging target in small cell lung cancer. J Hematol Oncol. 2019;12(1):61.
[6] Borghaei H, Paz‐Ares L, Johnson M, et al. OA12.05 Phase 1 updated exploration and first expansion data for DLL3‐targeted T‐cell engager tarlatamab in small cell lung cancer [abstract]. J Thorac Oncol. 2022;17(9 suppl):S33.
[7] Rudin CM, Hann CL, Garon EB, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012; 18(11): 3163-3169.
[8] Owonikoko TK, Niu H, Nackaerts K, et al. Randomized phase II study of paclitaxel plus alisertib versus paclitaxel plus placebo as second-line therapy for SCLC: primary and correlative biomarker analyses. J Thorac Oncol. 2020; 15(2): 274-287.
[9] Lau S C M, Pan Y, Velcheti V, et al. Squamous cell lung cancer: Current landscape and future therapeutic options[J]. Cancer Cell, 2022.
[10] Spigel DR, Reckamp KL, Rizvi NA, et al. A phase III study (CheckMate 017) of nivolumab (NIVO; anti-programmed death-1 [PD-1]) vs docetaxel (DOC) in previously treated advanced or metastatic squamous (SQ) cell non-small cell lung cancer (NSCLC). 2015 ASCO Abstract 8009.
[11] Gou LY, Wu YL. Prevalence of driver mutations in non-small-cell lung cancers in the People’s Republic of China. Lung Cancer (Auckl). 2014;5:1-9.
[12] Soria JC, Felip E, Cobo M, et al. Afatinib versus Erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): An open-label randomised controlled phase 3 trial[J]. Lancet Oncol, 2015, 16(8): 897-907.
[13] New Treatment Options in Advanced Squamous Cell Lung Cancer.2019 Jan;39:e198-e206.
Approval Number: DSCN-20231023-00001
*”The Medical Community” strives to publish content that is professional and reliable, but does not guarantee the accuracy of the content; relevant parties should verify independently when using or making decisions based on this information.