 This year, CED released the draft for “Technical Guidelines for Non-Clinical Research of Antibody-Drug Conjugates” to assist companies in conducting non-clinical research on ADCs.It provides guidance on non-clinical studies such as “pharmacology, safety pharmacology, pharmacokinetics, and toxicology.”(1) PharmacologyIn the early development of ADCs, studies on the in vitro and in vivo pharmacological/efficacy effects of ADCs should be conducted.1) In vitro pharmacological testing: It mainly includes the binding activity of ADC antibodies to cell surface receptors, the endocytosis of ADC-antigen complexes, the effects of ADC drugs on cell physiological morphology, and the evaluation of cell-killing effects.T-Dxd in vitro pharmacological testing experiment
This year, CED released the draft for “Technical Guidelines for Non-Clinical Research of Antibody-Drug Conjugates” to assist companies in conducting non-clinical research on ADCs.It provides guidance on non-clinical studies such as “pharmacology, safety pharmacology, pharmacokinetics, and toxicology.”(1) PharmacologyIn the early development of ADCs, studies on the in vitro and in vivo pharmacological/efficacy effects of ADCs should be conducted.1) In vitro pharmacological testing: It mainly includes the binding activity of ADC antibodies to cell surface receptors, the endocytosis of ADC-antigen complexes, the effects of ADC drugs on cell physiological morphology, and the evaluation of cell-killing effects.T-Dxd in vitro pharmacological testing experiment 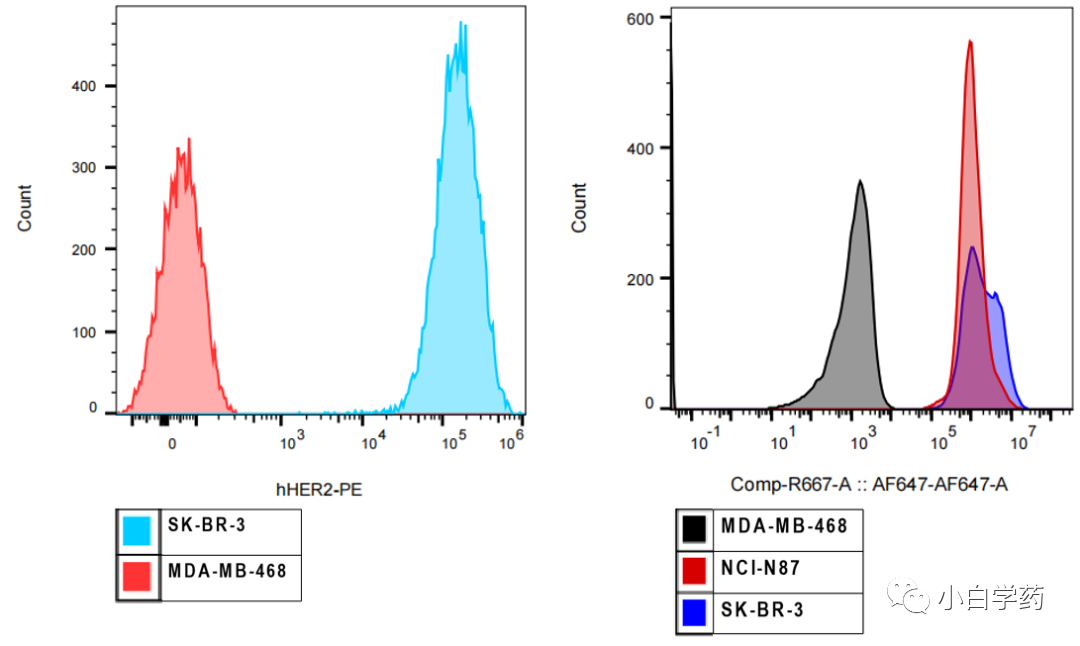 Figure T-DXd binding ability to cell surface receptors
Figure T-DXd binding ability to cell surface receptors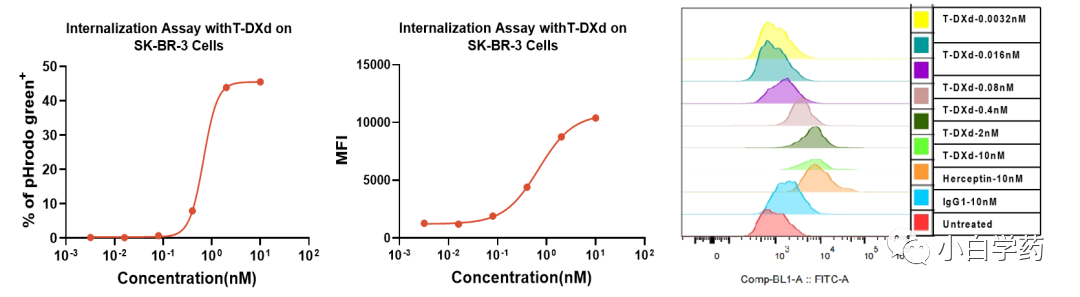 Figure T-DXd endocytosis
Figure T-DXd endocytosis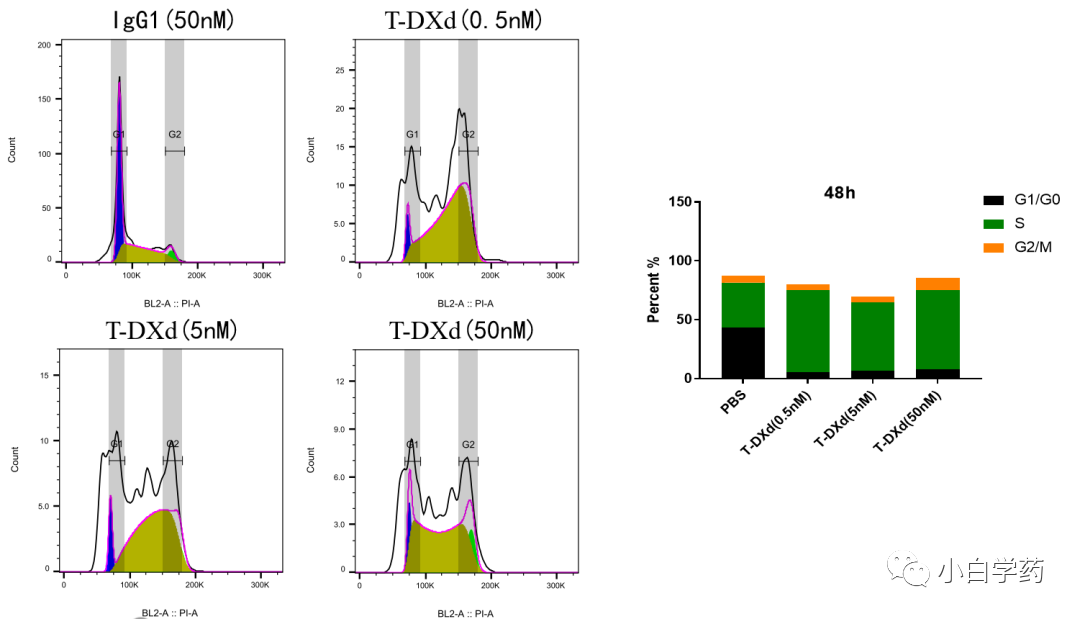 Figure T-DXd inhibits SK-BR-3 cell cycle
Figure T-DXd inhibits SK-BR-3 cell cycle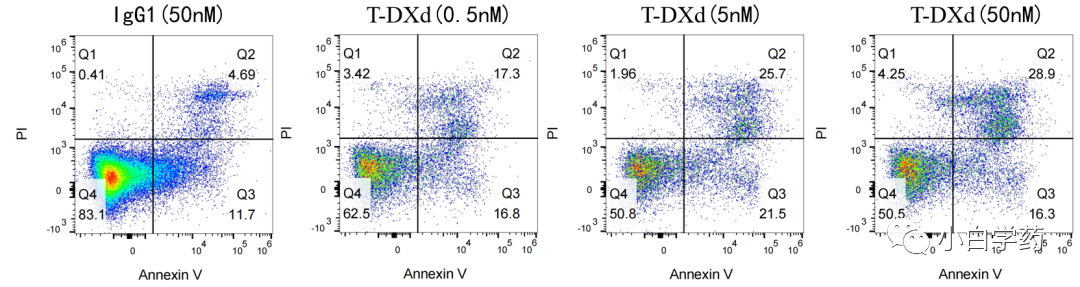 Figure T-DXd induces SK-BR-3 cell apoptosis
Figure T-DXd induces SK-BR-3 cell apoptosis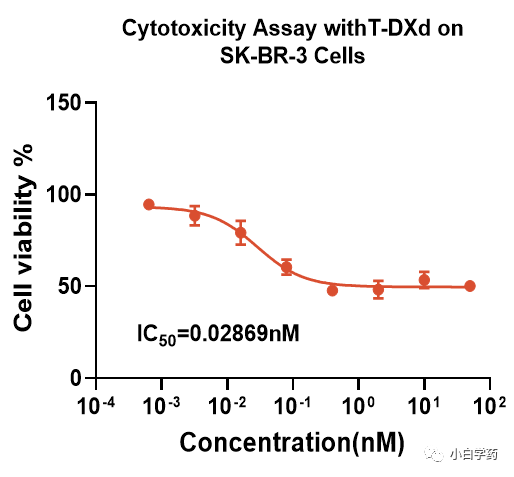 Figure T-DXd cell-killing effect in SK-BR-3 cells2) In vivo pharmacological testing: Depending on the method of administration and target, there are different model selections and experimental design standards for the in vivo efficacy evaluation of ADC drugs. In vivo experiments assess the tumor suppression effect of the drug T-DXd.ADC monotherapy: Generally, severe immunodeficient mice (NCG) can be used to establish tumor cell line xenografts (CDX) for related studies.ADC combination therapy: The combination of ADC drugs with anti-PD-1 monoclonal antibodies can produce bifunctional PD-1-targeted ADCs, which have greater application prospects.
Figure T-DXd cell-killing effect in SK-BR-3 cells2) In vivo pharmacological testing: Depending on the method of administration and target, there are different model selections and experimental design standards for the in vivo efficacy evaluation of ADC drugs. In vivo experiments assess the tumor suppression effect of the drug T-DXd.ADC monotherapy: Generally, severe immunodeficient mice (NCG) can be used to establish tumor cell line xenografts (CDX) for related studies.ADC combination therapy: The combination of ADC drugs with anti-PD-1 monoclonal antibodies can produce bifunctional PD-1-targeted ADCs, which have greater application prospects.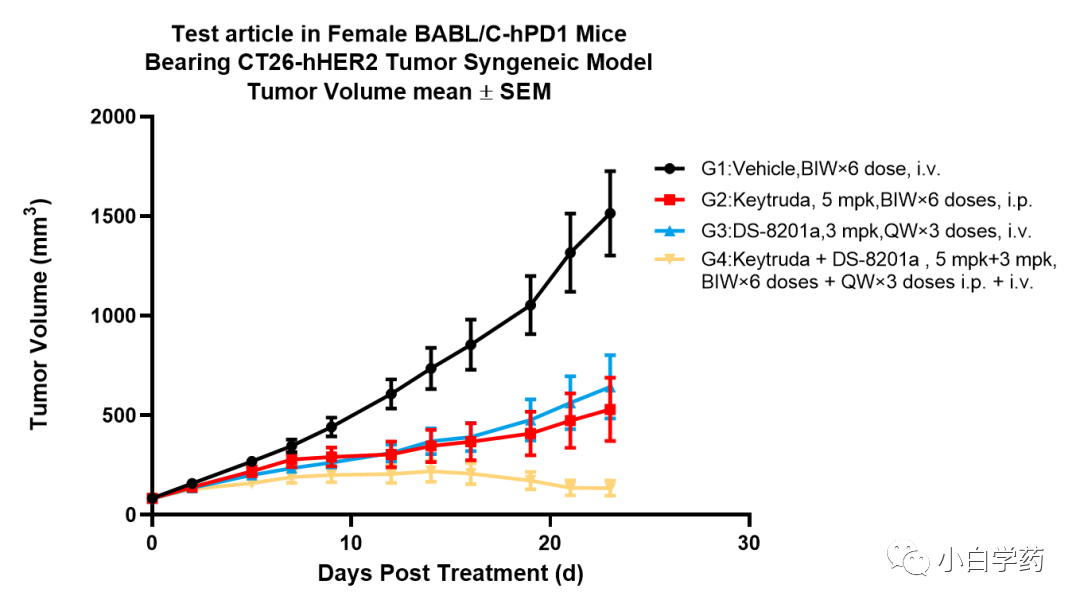 Figure In vivo efficacy test based on BALB/c-hPD1Experiments have shown that the PD-1 inhibitor Keytruda combined with the conjugate T-DXd (DS-8201a) exhibits stronger tumor suppression capabilities.Typically, in addition to the overall pharmacological/efficacy effects of ADCs, it is also necessary to study the pharmacological effects of each component. This mainly includes target antigen binding activity, possible pharmacological effects related to target antigens, and Fc effects; and the mechanism of action of free small molecule compounds or major pharmacologically active metabolites should be studied.Attention should be paid to the pharmacological differences between ADCs and naked antibodies, free small molecule compounds, or pharmacologically active metabolites, as well as the impact of antigen expression levels on pharmacological effects.(2) Safety PharmacologyBefore clinical trials, information on the impact of ADCs on important system functions must be obtained. Safety pharmacology tests can be conducted separately or combined with general toxicology tests.When free small molecule compounds are new compounds, separate tests are required to evaluate the potential effects of the drug on QT interval prolongation. If necessary, additional and/or supplementary safety pharmacology tests for ADCs and/or small molecule compounds should be conducted.ADCs have unique characteristics, one of which is PK, which significantly affects the half-life, clearance, elimination, and biodistribution of unbound components. Unbound antibodies involve metabolic degradation and target-mediated clearance pathways, while small molecule payloads are typically cleared through liver and kidney pathways.The clearance of ADCs involves the properties of both macromolecules and small molecules. The conjugate first undergoes metabolic degradation to produce ADC metabolites or deconjugation to produce naked antibodies and payload components. Once degraded, antibodies are broken down into amino acids for circulation, while payloads undergo renal and hepatic clearance. This difference in elimination affects the half-life of ADCs.Predicting potential safety risks in clinical settings is the goal of non-clinical toxicology research. Non-clinical safety studies of ADCs can enhance their predictive value by appropriately selecting animal test species, understanding the mechanisms of adverse reactions, or identifying the toxic driving factors of ADCs.Due to the presence of antibody structures, ADC drugs also carry a potential risk of inducing immunogenic reactions. The formation of anti-drug antibodies (ADA) can have certain effects on the pharmacological and/or toxicological actions of ADCs.The formation of ADA can impact pharmacokinetic (PK) parameters, the incidence and severity of toxic reactions, and long-term efficacy (due to the production of neutralizing antibodies).(3) ToxicologyIn 2000, the first-generation ADC drug Mylotarg developed by Pfizer was approved by the FDA but was withdrawn from the market in 2010 due to toxic side effects.Toxicology includes: 1. General toxicology 2. Genetic toxicity 3. Reproductive toxicity 4. Carcinogenicity 5. Immunogenicity/Immunotoxicity 6. Phototoxicity 7. Tissue cross-reactivity 8. Formulation safety 9. ToxicokineticsKey toxicology studies should be conducted in relevant species to assess potential safety risks both on-target and off-target.Currently, the safety evaluation of second and third-generation ADCs mainly includes on-target toxicity and off-target toxicity.On-target toxicity occurs when ADCs bind to target antigens on normal cells, so safety evaluations need to consider using animal species that exhibit similar characteristics to humans and have non-target tissue cross-reactivity.Off-target toxicity is mainly due to the instability of the conjugate, leading to normal cells non-specifically taking up the conjugated small molecule drugs (mainly mediated by Fc receptor-mediated endocytosis, binding with FcRn, and pinocytosis).Conducting safety evaluations in animal species without tissue cross-reactivity can help predict off-target toxicity to some extent and can serve as important support for improving the success rate of ADC development.Research should include toxicokinetic analysis of both conjugated and unconjugated components to assess the impact of stability on safety.Currently, there are two directions for the development of Payloads in the industry: one is high DAR value with low toxicity, and the other is low DAR value with high toxicity; the choice should be based on specific experimental data.Book RecommendationDrug Discovery: From Bedside to Wall Street
Figure In vivo efficacy test based on BALB/c-hPD1Experiments have shown that the PD-1 inhibitor Keytruda combined with the conjugate T-DXd (DS-8201a) exhibits stronger tumor suppression capabilities.Typically, in addition to the overall pharmacological/efficacy effects of ADCs, it is also necessary to study the pharmacological effects of each component. This mainly includes target antigen binding activity, possible pharmacological effects related to target antigens, and Fc effects; and the mechanism of action of free small molecule compounds or major pharmacologically active metabolites should be studied.Attention should be paid to the pharmacological differences between ADCs and naked antibodies, free small molecule compounds, or pharmacologically active metabolites, as well as the impact of antigen expression levels on pharmacological effects.(2) Safety PharmacologyBefore clinical trials, information on the impact of ADCs on important system functions must be obtained. Safety pharmacology tests can be conducted separately or combined with general toxicology tests.When free small molecule compounds are new compounds, separate tests are required to evaluate the potential effects of the drug on QT interval prolongation. If necessary, additional and/or supplementary safety pharmacology tests for ADCs and/or small molecule compounds should be conducted.ADCs have unique characteristics, one of which is PK, which significantly affects the half-life, clearance, elimination, and biodistribution of unbound components. Unbound antibodies involve metabolic degradation and target-mediated clearance pathways, while small molecule payloads are typically cleared through liver and kidney pathways.The clearance of ADCs involves the properties of both macromolecules and small molecules. The conjugate first undergoes metabolic degradation to produce ADC metabolites or deconjugation to produce naked antibodies and payload components. Once degraded, antibodies are broken down into amino acids for circulation, while payloads undergo renal and hepatic clearance. This difference in elimination affects the half-life of ADCs.Predicting potential safety risks in clinical settings is the goal of non-clinical toxicology research. Non-clinical safety studies of ADCs can enhance their predictive value by appropriately selecting animal test species, understanding the mechanisms of adverse reactions, or identifying the toxic driving factors of ADCs.Due to the presence of antibody structures, ADC drugs also carry a potential risk of inducing immunogenic reactions. The formation of anti-drug antibodies (ADA) can have certain effects on the pharmacological and/or toxicological actions of ADCs.The formation of ADA can impact pharmacokinetic (PK) parameters, the incidence and severity of toxic reactions, and long-term efficacy (due to the production of neutralizing antibodies).(3) ToxicologyIn 2000, the first-generation ADC drug Mylotarg developed by Pfizer was approved by the FDA but was withdrawn from the market in 2010 due to toxic side effects.Toxicology includes: 1. General toxicology 2. Genetic toxicity 3. Reproductive toxicity 4. Carcinogenicity 5. Immunogenicity/Immunotoxicity 6. Phototoxicity 7. Tissue cross-reactivity 8. Formulation safety 9. ToxicokineticsKey toxicology studies should be conducted in relevant species to assess potential safety risks both on-target and off-target.Currently, the safety evaluation of second and third-generation ADCs mainly includes on-target toxicity and off-target toxicity.On-target toxicity occurs when ADCs bind to target antigens on normal cells, so safety evaluations need to consider using animal species that exhibit similar characteristics to humans and have non-target tissue cross-reactivity.Off-target toxicity is mainly due to the instability of the conjugate, leading to normal cells non-specifically taking up the conjugated small molecule drugs (mainly mediated by Fc receptor-mediated endocytosis, binding with FcRn, and pinocytosis).Conducting safety evaluations in animal species without tissue cross-reactivity can help predict off-target toxicity to some extent and can serve as important support for improving the success rate of ADC development.Research should include toxicokinetic analysis of both conjugated and unconjugated components to assess the impact of stability on safety.Currently, there are two directions for the development of Payloads in the industry: one is high DAR value with low toxicity, and the other is low DAR value with high toxicity; the choice should be based on specific experimental data.Book RecommendationDrug Discovery: From Bedside to Wall Street

This book tells the story of drug development using real cases based on current scientific research and events, objectively and clearly describing the successes, failures, shortcomings, and limitations of the biopharmaceutical industry. It offers insights into the development of new drugs for various conditions such as cancer and pain. It presents a fair and unbiased discussion on how to better translate basic science into drug discovery.
References[1] National Medical Products Administration Drug Review Center “Technical Guidelines for Non-Clinical Research of Antibody-Drug Conjugates (Draft for Comments)”[2] www.biomart.cn/news/16/3067505.htm