Breast cancer, regarded as the “killer of beauty,” is a malignant tumor that severely threatens women’s health, with an increasing incidence year by year. Antibody-drug conjugates (ADC), as a new type of therapeutic drug, have become a hot research topic in the field of breast cancer due to their advantages of “high efficacy and low toxicity.” In recent years, more than 100 ADC drugs have been undergoing clinical trials globally, with 14 approved for market, and currently, 3 ADC drugs have been approved for the treatment of breast cancer in China, significantly impacting the treatment landscape of breast cancer. So, what are ADC drugs? What advantages do ADC drugs have? Which patients can use ADC drugs?

1
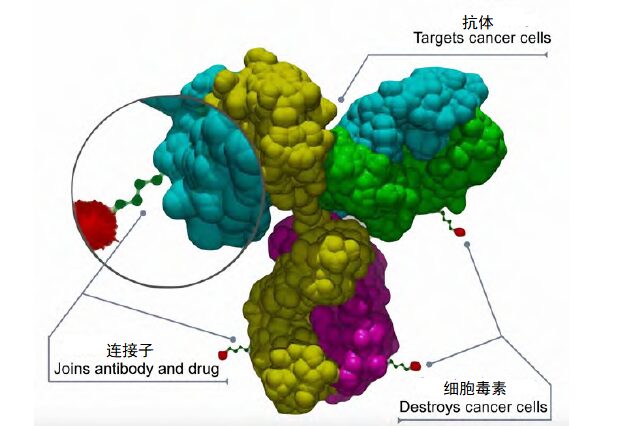
What Are ADC Drugs?
-
The antibody is the main component of ADC drugs, which has high specificity and can specifically bind to antigens on the surface of tumor cells.
-
The linker is the molecule that connects the antibody and the cytotoxin. Its main function is to maintain the stability of the ADC and release the cytotoxin after the antibody binds to the tumor cell.
-
The cytotoxin is the pharmacological component of ADC drugs, which has high cytotoxicity and can kill cancer cells. The cytotoxin can be a chemical drug, a radioactive nuclide, or other cytotoxic substances.
2
What Are the Advantages of ADC Drugs?
3
Which Breast Cancer Patients Can Receive ADC Drug Treatment?
Conclusion
References
[1] Chinese Anti-Cancer Association Clinical Research Committee of Anticancer Drugs, National Expert Committee for Monitoring Clinical Application of Anticancer Drugs, National Quality Control Center for Breast Cancer Experts, et al. Clinical Application of Antibody-Drug Conjugates in the Treatment of Malignant Tumors: Chinese Expert Consensus (2023 Edition) [J]. Chinese Journal of Oncology, 2023, 45(9):741-762.
[2] Yang Huixin, Qian Yingying, Huang Xu, et al. Research Progress of Anti-HER2 Breast Cancer ADC Drugs [J]. Chinese Journal of Geriatrics, 2023, 43(2):500-506.
[3] Zhuang Ming. “Magic Bullet” ADC Drugs—The Nemesis of Breast Cancer [J]. Science and Technology Vision, 2023, 7:25-28.
[4] Expert Group of “Chinese Breast Cancer Neoadjuvant Therapy Consensus (2022 Edition).” Chinese Breast Cancer Neoadjuvant Therapy Consensus (2022 Edition) [J]. Chinese Journal of Cancer, 2022, 32(1):80-89.
[5] Bardia, A., Hurvitz, S. A., Tolaney, S. M., Loirat, D., Punie, K., Oliveira, M., … & Rugo, H. S. (2021). Sacituzumab govitecan in metastatic triple-negative breast cancer. New England Journal of Medicine, 384(16), 1529-1541.
Editor: Yan Xiaoqian
Layout Editor: Awa