Since the first use of cytotoxic chemicals to treat cancer, oncologists have been searching for ways to enhance efficacy without significantly increasing overall toxicity to patients. One of the methods attempted to improve the killing effect on tumor cells is the use of cytotoxic agents with potency at picomolar or lower levels (such as microtubule inhibitors or DNA alkylating agents). However, these compounds lack a sufficient therapeutic window for cancer treatment.
The invention of monoclonal antibodies has provided the possibility of utilizing their strong specificity for binding as a mechanism to create antibody-drug conjugates (ADCs) through chemical coupling of cytotoxic effectors, thereby selectively delivering cytotoxic drugs to cancer cells.
Although this “simple concept” faces numerous challenges in translating into clinical practice, it has catalyzed active research in the field since the FDA approved Brentuximab vedotin (BV) in 2011 and Trastuzumab emtansine (T-DM1) in 2013.
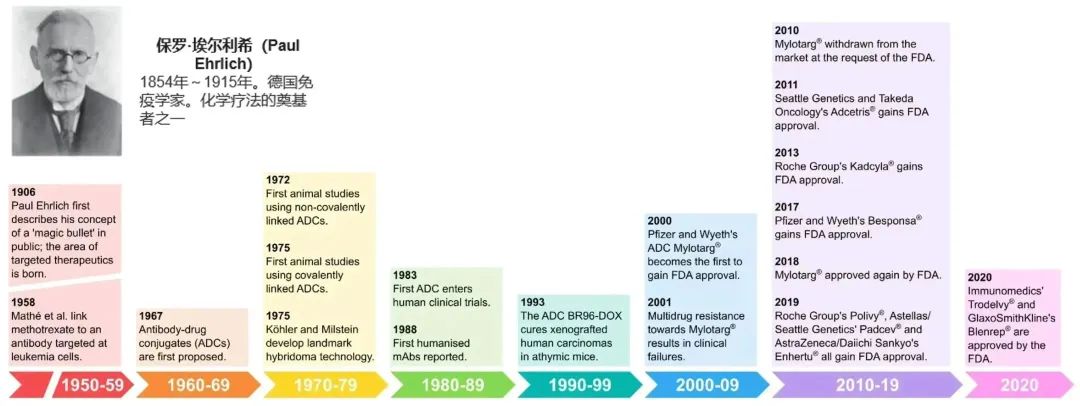
Figure 1. History and Milestones of ADC Drug Development (1906-2020)[1]
Overview of ADC Drug Background
1 Structure and Mechanism of Action
ADC molecules consist of a monoclonal antibody, a linker, and a payload (Figure 2).
Figure 2. Schematic Diagram of ADC Molecular Structure: The general structure of ADC molecules consists of a monoclonal antibody (gray) conjugated with a cleavable/non-cleavable linker (orange) and a cytotoxic payload (gold)[2]
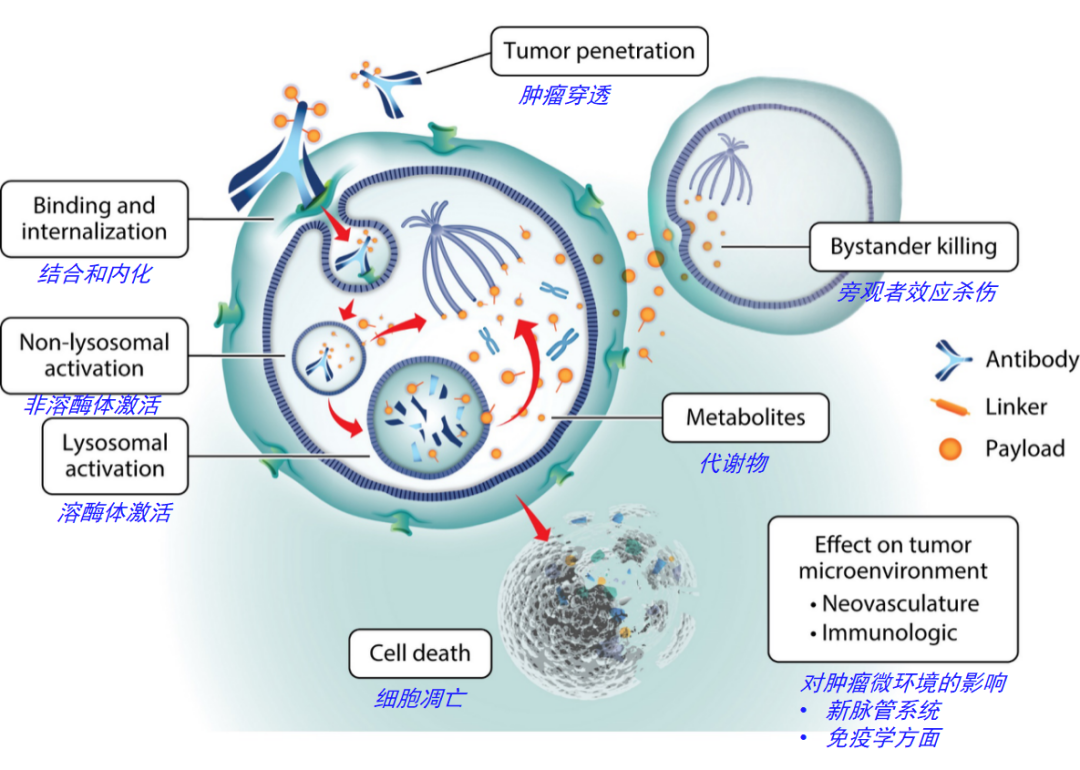
Figure 3 illustrates the primary mechanisms of action of ADC drugs. The ADC molecules enter tumor tissues from the vascular system, bind to surface targets on tumor cells, and then internalize via the endosomal-lysosomal pathway, where the linker is cleaved and/or the antibody is degraded to release the payload, which ultimately diffuses into the cytoplasm to reach its target (tubulin) to disrupt microtubule dynamics. Other widely used payloads, such as calicheamicin targeting DNA, must further diffuse from the cytoplasm to the nucleus (not shown in Figure 3)[3].
If the cytotoxic metabolites can freely pass through the cell membrane and diffuse, they can enter adjacent cells and exert a bystander effect.
Figure 3. Mechanism of ADC Action Equipped with Payloads (Auristatin, Maytansine)[3]
Considerations in Pharmaceutical Research of ADC Drugs
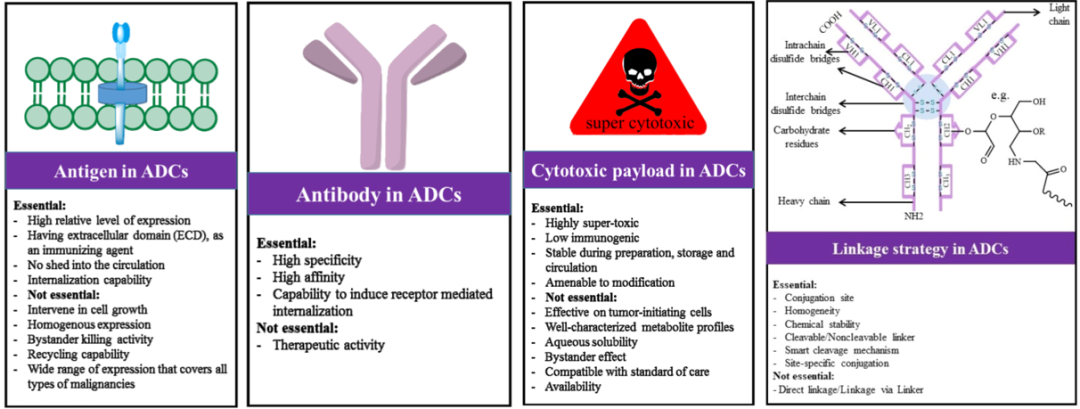
ADC molecules have a complex and sophisticated structure, and their molecular design needs to consider multiple factors and their interrelationships, such as antigen, antibody, payload, linker, and coupling strategy[7]. Various specific considerations are referenced in Figure 7.
Figure 7. Design Considerations for ADC Drugs[4]
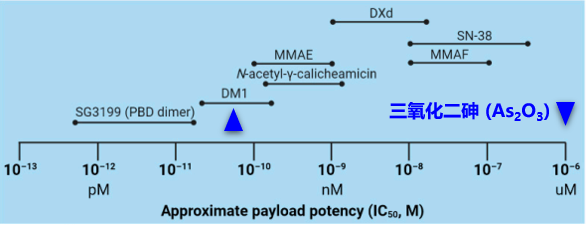
The payload is a critical component for the cytotoxic action of ADC, and it must meet several main requirements: (1) high cytotoxicity, typically with an IC50 value at low nanomolar or picomolar levels (see Figure 8); (2) clear targets and mechanisms of action; (3) (potential) chemical attachment sites.
Figure 8. Concentration Range of Common Payloads Used in ADC Drugs
Derivatives of Maytansine (DM1/DM4) or Auristatin (MMAE/MMAF) are commonly used microtubule inhibitors. Other types of cytotoxic drugs include enediynes (calicheamicin), derivatives of dolastatin, pyrrolobenzodiazepines (PBD), and indolyl-2-carboxamides, all targeting the minor groove of DNA; additionally, quinoline alkaloids (SN-38) can inhibit DNA topoisomerase I.
In 114 completed or ongoing clinical trials, the drug payloads used lack diversity, with only 7 payload formulations reported (4 additional trials are ongoing with structures not reported). Six of the seven payload mixtures come from natural product sources, indicating the key value of natural products in the types of ADC payloads[5,6].
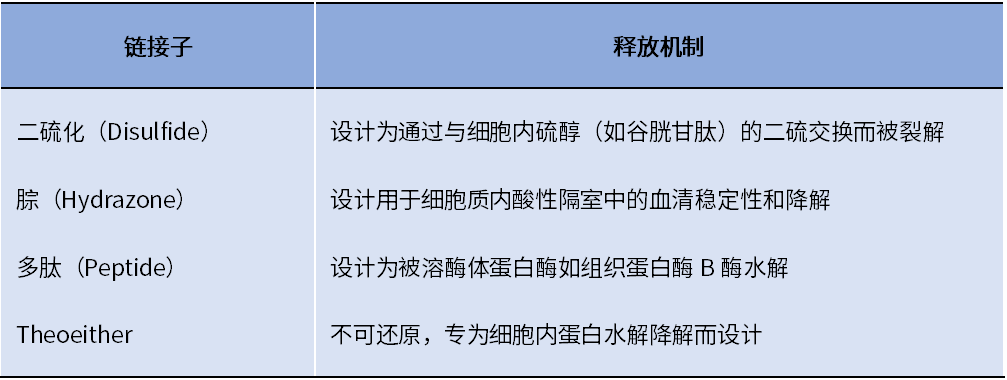
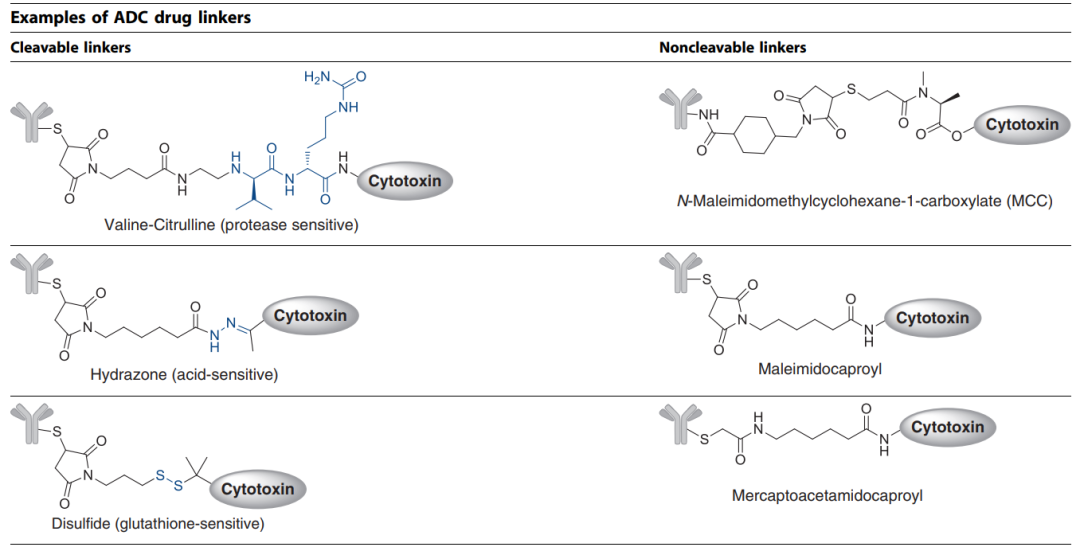
One of the biggest challenges in ADC development is selecting the appropriate linker to attach the cytotoxic payload to the antibody. The chemistry of the linker affects various properties of the ADC, including toxicity, specificity, stability, and potency. Common linker technologies and release mechanisms for ADCs are shown in Table 2.
Table 2. ADC Linker Technologies and Release Mechanisms
Linkers can be broadly categorized into cleavable (where the payload can separate from the antibody at the tumor site) or non-cleavable (where the payload and antibody remain bound, and the antibody degrades after internalization), as shown in Figure 9.
Figure 9. The Two Main Types of ADC Drug Linkers (Cleavable and Non-Cleavable)[7]
Common cleavable linkers include chemical reactive linkers, acid-labile linkers, reducible linkers, and enzyme-cleavable linkers. Non-cleavable linkers release cytotoxic payloads during the lysosomal degradation process of antibody-drug conjugates in the tumor environment, bypassing the non-specific dispersion of toxic formulations. One advantage of non-cleavable linkers is increased stability in plasma, reducing off-target toxicity.

Mylotarg® employs a hydrazone linker strategy that is unstable in acidic conditions. Theoretically, the hydrazone should be stable in blood circulation at physiological pH and undergo selective hydrolysis under more acidic conditions after internalization (the pH of endosomes is approximately 5.0–6.5 and that of lysosomes is 4.5–5.0). However, reports indicate that the linker of Mylotarg® exhibits some instability, leading to premature release of the payload in plasma circulation, potentially causing off-target toxicity. Benefiting from knowledge accumulated in clinical settings in recent years, Pfizer has reduced the dosing of this drug and modified the administration regimen, ultimately receiving FDA re-approval in 2017[2,8].
2.3 Drug-Antibody Ratio (DAR)
The average number of payload molecules per antibody is usually referred to as the drug-antibody ratio (DAR). The DAR determines the amount of “payload” that can be delivered to tumor cells and can directly affect safety and efficacy. A high drug load often leads to rapid plasma clearance, while ADCs with lower DAR exhibit weaker activity.
The successful design of clinical ADCs depends not only on the potency of the payload and its ratio, linker stability, and payload release, but also on the choice of coupling technology.
Over the past decade, all ADCs approved by the FDA have been composed of mixtures of ADCs with different numbers of drugs attached at various positions on monoclonal antibodies. A series of new coupling strategies have been developed in the industry aimed at controlling the attachment position and quantity of the payload while maintaining structural integrity and homogeneity.
Figure 10. Random and Site-Specific Coupling Strategies: Randomly coupled ADC products include chemical isomers (a), while site-specific coupling methods produce a relatively homogeneous product distribution (b)[7]
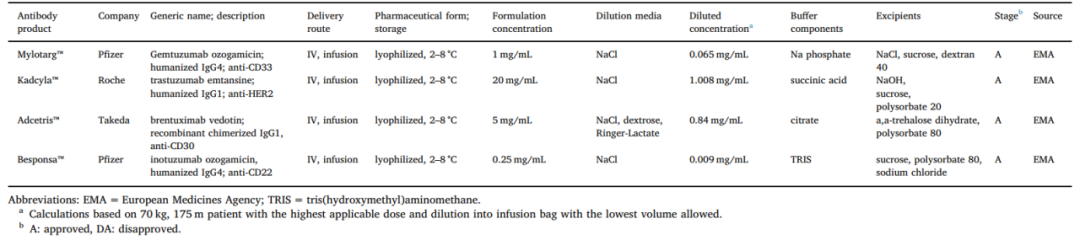
2.5 Stability of ADC Drugs[9]
The drug component concentration of marketed ADC formulations is often below 20 mg/mL, as its high selectivity and efficacy avoid the need for high concentrations. Lower concentrations reduce the risk of aggregation, which is particularly important for ADC molecules with hydrophobic payloads, as the solubility of ADCs may be lower compared to the monoclonal antibodies themselves. However, since ADC drugs are often administered via infusion in clinical settings, low ADC concentrations in infusion bags can also pose problems, especially as hydrophobic payloads increase the risk of drug loss due to adsorption to plastic materials.
Liquid formulations for ADC products may face stability issues, while lyophilized formulations can reduce linker degradation during storage. Almost all ADC drug formulations approved for marketing are lyophilized forms.
Another interesting phenomenon is that some ADC formulations may be more photosensitive than traditional antibodies, such as Mylotarg®, Besponsa®, and Enhertu®, which all have brown glass vials as their primary packaging.
Table 3. Formulation Information of Several Approved ADC Drugs
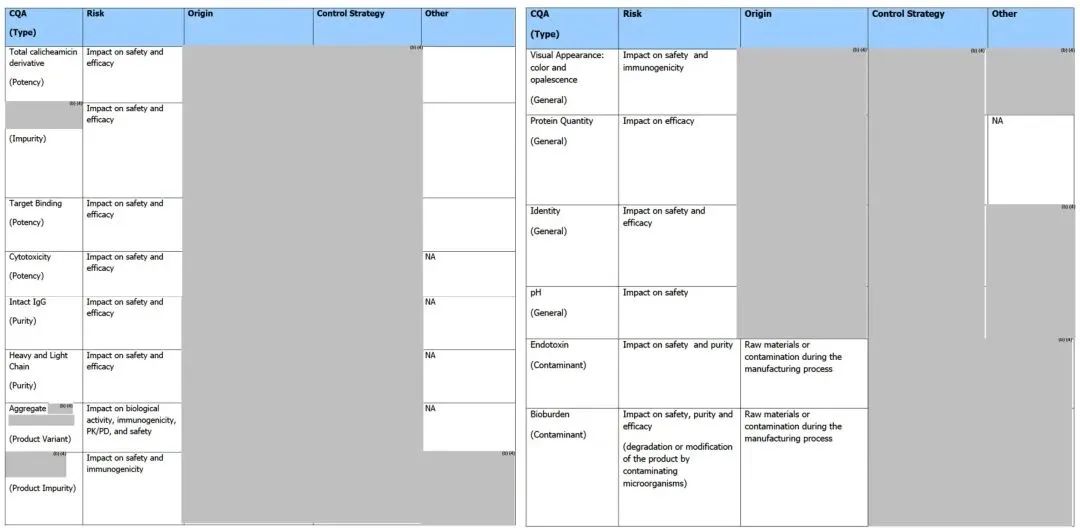
2.6 Critical Quality Attributes (CQA) of ADC Drugs
ICH Q8 R2 defines and explains critical quality attributes as “physical, chemical, biological, or microbiological properties or characteristics that should be within appropriate limits, ranges, or distributions to ensure the desired product quality (safety, efficacy).” Thus, understanding the CQA of ADC drugs is of great significance for ADC drug development. Some common critical quality attributes of ADC drugs are shown in Table 4.
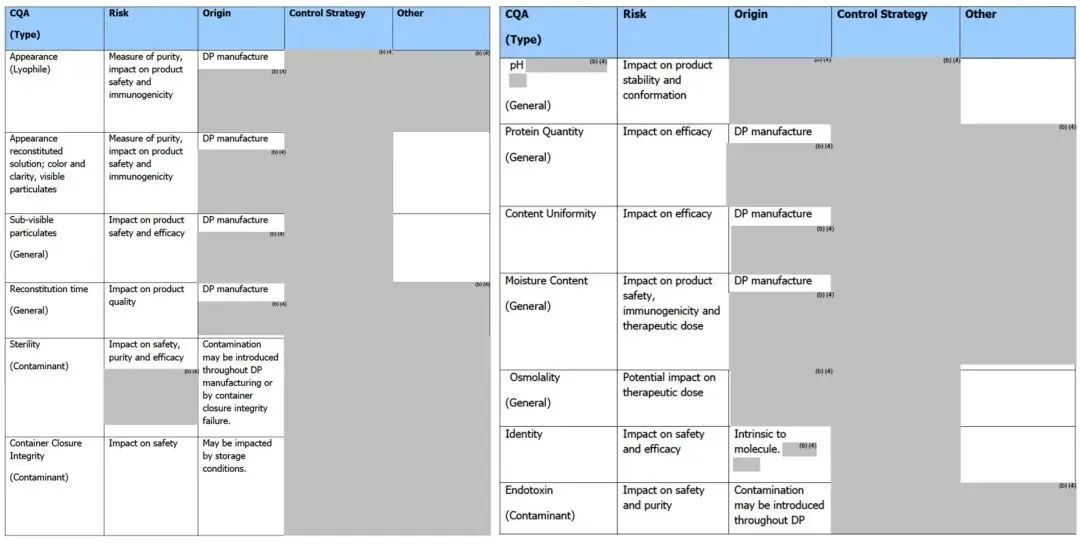
Table 4. Critical Quality Attributes of ADC Drugs (Part)[10]
Tables 5 and 6 present detailed CQA information of the marketed ADC product Mylotarg® for the reader’s reference.
Table 5. CQA of the Marketed ADC Product Mylotarg® (Bulk) (FDA Review Report: FDA Product Quality Review Report_BLA 761060 Mylotarg)
Table 6. CQA of the Marketed ADC Product Mylotarg® (Finished Product) (FDA Review Report: FDA Product Quality Review Report_BLA 761060 Mylotarg)
Moreover, unlike the information disclosed by the FDA, the EMA review report of Mylotarg® (EMA/155284/2018_ Mylotarg Assessment report) shows that its bulk quality standards include general properties, identification, purity, biological activity, product-related impurities, and safety tests. Additionally, the structural characterization of the bulk includes information on post-translational modifications, charge & size heterogeneity, conjugation sites, calicheamicin derivative conjugation levels, higher-order structure, and biological activity.
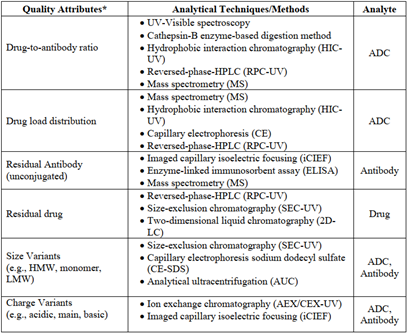
2.7 Analytical Techniques Related to ADC Drugs
Generally, analytical tools used for conventional antibody drugs may also apply to ADC drug analysis, but not all methods can be seamlessly interchangeable. For instance, if the ADC’s payload has UV absorption or the sample is highly heterogeneous; or in size exclusion chromatography (SEC), if the ADC has a hydrophobic payload structure, it may require the addition of organic solvents to the mobile phase to reduce strong hydrophobic interactions with the chromatography column.
Below are several unique quality attribute analyses for ADC drugs:
Determination of Drug-Antibody Ratio (DAR)
UV-visible spectroscopy is a simple and practical method for determination, but it has prerequisites: 1) The payload should contain a UV-visible chromophore; 2) The payload and antibody should exhibit distinct and separated maximum absorption peaks in their UV-visible spectra; 3) The presence of the payload should not affect the absorption characteristics of the antibody portion, and vice versa.
Hydrophobic interaction chromatography (HIC) relies on the number of hydrophobic payloads attached to the antibody to separate conjugates. The least hydrophobic unconjugated antibodies elute from the column first, while the conjugates with the highest DAR elute last. Besides DAR, this method can also provide the conjugation level of the ADC, making it a classic chromatographic technique for ADC drug analysis.
Mass spectrometry is a powerful technique for characterizing ADC structures, capable of separating, identifying, and quantifying molecular species, and can also be used to determine DAR.
Determination of Payload Distribution
The overall payload distribution can be analyzed through mass spectrometry of the intact molecular weight, and for ADCs with less heterogeneity produced by cysteine or other site-specific coupling, hydrophobic interaction chromatography can also be used for characterization. To determine the payload distribution at each conjugation site, more comprehensive analyses, such as reductive mass spectrometry peptide mapping, are required.
Reversed-phase high-performance liquid chromatography (RPC) or capillary electrophoresis (CE) can provide information about the distribution of the payload on light and heavy chains under reducing conditions as orthogonal testing methods.
Determination of Unconjugated Antibodies
The level of unconjugated antibodies is a key parameter in process control, as it can directly affect the efficacy of ADC drugs. Mass spectrometry is a common tool for quantifying unconjugated antibodies. Due to the inherent lipophilicity of the payload or linker, the binding of hydrophobic (lipophilic) payloads can also change the hydrophobicity of the protein molecule, thus hydrophobic interaction chromatography can also be used to determine the level of unconjugated antibodies in ADC samples.
Since the binding of the payload to the antibody can greatly alter the surface and overall charge distribution, techniques such as intact column imaging capillary isoelectric focusing (icIEF) are also available as analytical tools. Other methods worth attempting include: reversed-phase high-performance liquid chromatography, capillary electrophoresis, enzyme-linked immunosorbent assay (ELISA), etc.
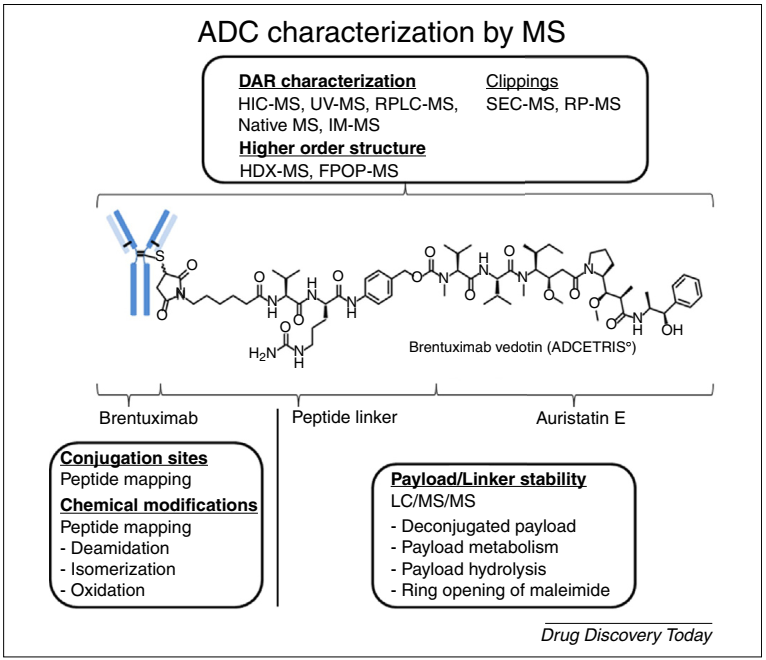
It is worth mentioning that mass spectrometric analysis techniques are powerful tools for structural characterization of ADC drugs, playing an indispensable role in ADC drug process development and quality analysis, with common uses of mass spectrometry in ADC drug quality analysis detailed in Figure 11.
Figure 11. Strategies for Structural Characterization of ADC Based on Mass Spectrometry: In-depth characterization can be performed on intact ADCs, monoclonal antibodies, cytotoxic payloads, or linker structures[11]
2.8 Process Development and Production of ADC Drugs[11]
Compared to any other active pharmaceutical ingredient (API) category, the quality of ADC drugs is more constrained by the production process. A well-designed production process is essential for the reproducible production of a heterogeneous subset of compounds. To ensure process consistency, numerous parameters of the coupling process must be studied and controlled. The selection of appropriate processing, storage, and handling conditions during production should be based on the physicochemical stability study data of intermediates and ADCs.
Refined process development also requires experienced and well-trained personnel, as well as appropriate experimental equipment.
To conduct more experiments with minimal materials, micro-jacketed vessels that allow for precise temperature control have proven very useful for coupling experiments at milligram levels, and this reaction model has been shown to simulate reaction performance up to several hundred liters; however, for purification processes, gram-scale operations are required to evaluate tangential flow filtration (TFF) conditions.
The biggest challenge in ADC drug production may be the design, construction, and operation of a biomanufacturing environment that allows for the safe handling of potent cytotoxic drugs and their contaminants (e.g., the transfer of materials containing toxic substances and disposal of contaminants). Therefore, personnel training should be strengthened to ensure the proper use of equipment and adherence to safety concepts; additionally, a personal protective equipment plan should be developed, covering residual risks that engineering controls cannot eliminate and emergency plans for toxic compound leaks.
Cleaning of equipment is another critical activity when manufacturing potent cytotoxic drugs. Disposable technologies can reduce cleaning operation costs, but materials are often expensive, and leachability and extractability data may also be required. Therefore, glass or stainless steel containers are typically preferred for coupling reactions.
Cleaning validation of ADC production lines requires high-sensitivity detection methods, as traditional TOC and HPLC methods for detecting residual cytotoxins often do not provide sufficient sensitivity, and validated ELISA or LC-MS analytical methods may be needed.
Prospects and Challenges of ADC Drugs
Despite the complex design of ADCs, the prospects of this therapeutic topic have garnered great interest in recent decades. The strong pipeline of ongoing clinical research and the continuous approval of ADC drugs are the best examples of promising prospects[7]. In addition to their current widespread application in the field of tumor treatment, the concept of ADCs also has potential applications in other therapeutic areas, such as treating methicillin-resistant Staphylococcus aureus infections, vancomycin-resistant intracellular Staphylococcus aureus infections, or anti-inflammatory conditions[1].
The preclinical lead selection methods for current ADC candidates typically rely on systematic in vitro assessments of various antibodies, linkers, and cytotoxic payloads, while it remains to be seen whether in vitro models are sufficient to predict drug responses. Early in vivo studies may be crucial before further understanding of ADCs[7].
One of the major challenges of ADC drugs is off-target toxicities, which result from premature release of cytotoxic small molecules into the bloodstream. Clinically reported off-target toxicities include hepatotoxicity, thrombocytopenia, peripheral neuropathy, neutropenia, anemia, and ocular toxicity. In addition, the complex potential mechanisms of drug resistance associated with ADCs remain to be elucidated; and identifying resistance mechanisms to ADCs in the context of therapeutic combinations with other administered compounds poses significant challenges[7,8,13].
Despite the numerous challenges in the design, process, and product development of ADC drugs, as well as their clinical practice, more clinical trials and basic research on existing ADCs will pave the way for addressing issues related to tumor markers, antibodies, cytotoxic payloads, and coupling strategies, making the future of ADC drugs promising.

[1] Antibody–Drug Conjugates—A Tutorial Review. Molecules 2021, 26, 2943
[2] Antibody-Drug Conjugate-Based Therapeutics: State of the Science. JNCI J Natl Cancer Inst (2019) 111(6): djz035
[3] Antibody–Drug Conjugates for Cancer Treatment. Annu. Rev. Med. 2018. 69:191–207
[4] Antibody-Drug Conjugates: Possibilities and Challenges. Avicenna Journal of Medical Biotechnology, Vol. 11, No. 1, January-March 2019
[5] Introduction to Antibody-Drug Conjugates. Antibodies 2021, 10, 42
[6] An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules 2021, 26, 5847
[7] Antibody–drug conjugates: current status and future directions. Drug Discovery Today, Volume 00, Number 00, Dec 2013
[8] Antibody–Drug Conjugates: The Last Decade. Pharmaceuticals 2020, 13, 245
[9] Antibody-drug conjugates- stability and formulation. European Journal of Pharmaceutics and Biopharmaceutics 139 (2019) 168–176
[10] Challenges and new frontiers in analytical characterization of antibody-drug conjugates. MAbs. 2018 Feb/Mar;10(2):222-243
[11] Characterization of antibody–drug conjugates by mass spectrometry: advances and future trends. Drug Discovery Today, Volume 00, Number 00, April 2016
[12] Chapter 29: Challenges in the Development and Manufacturing of Antibody–Drug Conjugates,489-497
[13] Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines 2021, 9, 872
Statement and Copyright Notice
Statement: This article aims to convey information on industry development and explore cutting-edge progress in biomedicine. The content of the article represents the author’s views and does not represent the position of eMedClub, nor does it constitute any value judgment, investment advice, or medical guidance. For any needs, please consult professionals for investment or visit a regular hospital for treatment.
Copyright Notice: This article is from the eMedClub content team, and personal forwarding to WeChat Moments is welcome. Unauthorized media or organizations are prohibited from reprinting in any form to other platforms. For reprint authorization, please leave a message below the article to obtain.