


Introduction
Despite rapid advancements in targeted therapy for NSCLC (non-small cell lung cancer), there remains an unmet need. This article summarizes the latest progress in targeted therapies for NSCLC involving Her2, Her3, and Trop-2 to inform readers.
 NSCLC and ADC
NSCLC and ADC
Whether through targeted therapy or immunotherapy, the number of lung cancer patients benefiting remains limited, and most lung cancer patients still develop acquired resistance. Therefore, research into alternative treatment methods is extremely important. In recent years, with the rapid development of ADC (antibody-drug conjugates), promising efficacy has been shown in lung cancer, primarily targeting Her2, Her3, and Trop-2. (🔗 Review: ADC Drugs; Understanding ADCs in One Image)
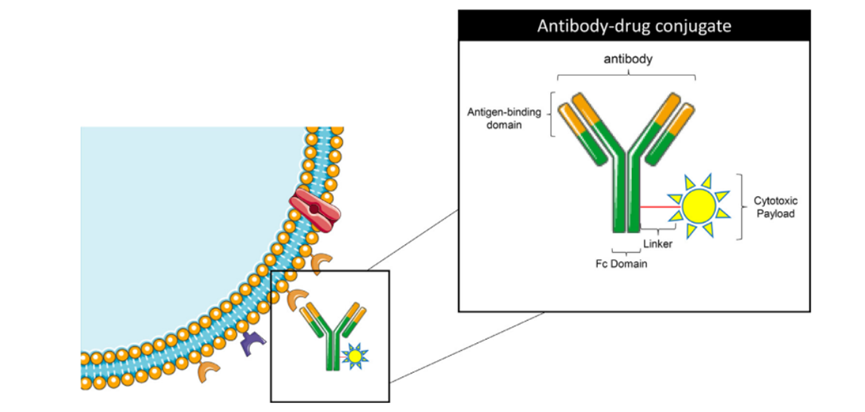 Figure 1: Schematic Structure of Antibody-Drug Conjugates [1]
Figure 1: Schematic Structure of Antibody-Drug Conjugates [1]

Figure 2: ADC Drugs Under Development in Lung Cancer [1] HER2 ADC
HER2 ADC
Among the ADC drugs being researched in lung cancer, the target HER2 (human epidermal growth factor receptor 2, also known as ERBB2) is the most prevalent.
HER2 ADC has also dominated the development of ADC drugs, but mutations in Her2 are primarily concentrated in the fields of breast cancer and gastric cancer, with a lower mutation rate in lung cancer, leading to slower research progress. However, mutations in HER2 are closely related to increased disease recurrence and poor prognosis, making treatment for this patient group a clinical challenge worth investigating.Statistics show that approximately 3% of NSCLC patients have HER2 mutations, with 90% of these mutations being exon 20 mutations, as illustrated below.

Figure 3: Schematic Structure of Her2 [2]
The ADC drugs targeting the Her2 receptor that have made the fastest progress are T-DM1 and DS-8201 (T-DXd).
T-DM1 is an ADC drug developed by Roche, with a drug-antibody ratio (DAR) of 3.5. The antibody used is a monoclonal antibody targeting HER2—Trastuzumab, and the linked chemotherapeutic agent is Emtansine (a chemotherapy drug that inhibits microtubule assembly). Emtansine has significant side effects when administered intravenously and is rarely used clinically. However, through the molecular design of ADC drugs, an antibody-conjugated structure is formed, allowing for localized release in tumor cells, which can exert excellent anti-cancer effects while effectively reducing toxicity. Currently, it has become the standard second-line treatment for HER2-positive breast cancer. In lung cancer, a Phase II basket clinical trial of T-DM1 for HER2 mutation-positive lung cancer patients has been initiated, involving 18 patients with metastatic HER2 mutation-positive lung adenocarcinoma, half of whom had previously received HER2-targeted therapy. The final results are encouraging:
- The response rate to T-DM1 was approximately 44%, all of which were partial responses;
- The response time point for T-DM1 was 1-4 months, with the longest progression-free survival after response being >11 months.
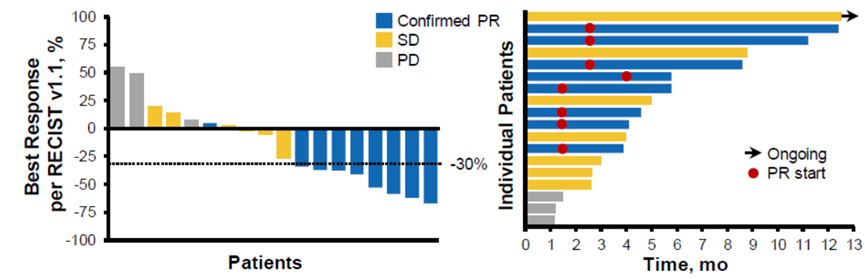 Figure 4: T-DM1 Lung Cancer Phase II Results
Figure 4: T-DM1 Lung Cancer Phase II Results
This is the first clinical trial to show positive results in the molecular subgroup of HER2-positive lung cancer.
With further optimization of ADC technology, the research momentum of DS-8201 developed by AstraZeneca/Daiichi Sankyo has recently surpassed that of T-DM1. In the latest NCCN breast cancer guidelines, DS-8201 is now recommended at a higher level than T-DM1 for HER2-positive breast cancer. What about its progress in HER2-positive lung cancer?

Figure 5: Schematic Structure of T-DXd
DESTINY-Lung01 is a Phase II study of T-DXd for treating HER2 mutation-positive lung cancer patients. Among the 42 enrolled patients, the overall response rate (ORR) reached 62% (26/42), with a median progression-free survival (PFS) of 14 months. Based on the good efficacy of T-DXd, on May 18, 2020, the FDA granted T-DXd breakthrough therapy designation for the treatment of metastatic HER2 mutation-positive NSCLC patients. We look forward to results from larger sample sizes.
Both T-DM1 and DS-8201 have confirmed that HER2-targeted ADCs are effective for HER2-positive lung cancer patients. But can HER3-targeted therapies also provide benefits?  HER3 ADC
HER3 ADC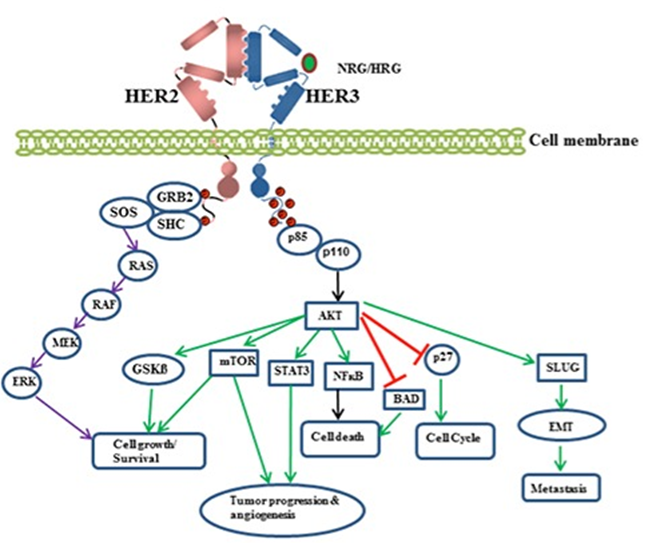 Figure 6: HER2/HER3 Heterodimer Activation Related Downstream Signaling Pathways [3]
Figure 6: HER2/HER3 Heterodimer Activation Related Downstream Signaling Pathways [3]
It is worth mentioning that, HER2 is a mature target for tumor therapy, and to date, no ligands that can directly bind to HER2 have been found in the human body. HER2 must form homodimers or heterodimers with other family members (such as HER3). Upon dimerization, HER2 undergoes a conformational change, activating intracellular tyrosine kinase activity, which in turn activates downstream signaling nodes (MAPK signaling pathway and PI3K/AKT signaling pathway), thereby exerting corresponding physiological effects. Additionally, HER3 is expressed in the gastrointestinal tract, reproductive system, skin, nervous system, urinary tract, and endocrine system of normal adults, and overexpression of HER3 protein is associated with many cancers, including prostate cancer, bladder cancer, and breast cancer.
Based on this, many research institutions have shifted their focus from the highly competitive HER2 to HER3, hoping that this target, which is in the same family as HER2, can also serve as a new target for tumor therapy. HER3 is expressed in 83% of NSCLC tumors, and overexpression of HER3 is associated with metastatic progression and reduced disease-free survival rates in NSCLC patients, as well as being related to various EGFR-TKI resistance mechanisms. Therefore, if breakthroughs can be made with HER3-targeted therapies in lung cancer, the prospects will be limitless.

Figure 7: Schematic Structure of HER3-DXd
The fastest progress is being made with the HER3-DXd developed by Daiichi Sankyo, which has entered Phase I clinical trials in lung cancer involving advanced NSCLC patients who have previously received EGFR-TKI treatment. A total of 57 patients received HER3-DXd treatment at a dose of 5.6 mg/kg every three weeks. The ORR for this group of patients was 39%, with a median duration of response (DOR) of 6.9 months, a median follow-up period of 10.2 months, and a median PFS of 8.2 months, with treatment responses observed in patients with various resistance mechanisms.
In addition to lung cancer, explorations of HER3-DXd in breast cancer and colorectal cancer are also ongoing, which is promising.
TROP-2 ADC

Figure 8: TROP2 Promotes Tumor Infiltration and Metastasis (Image Source: Cusabio)
In recent years, research on Trop2 (human trophoblast cell surface antigen 2) has also been increasing. It is an important tumor development factor, highly expressed in various tumors such as breast cancer, gastric cancer, non-small cell lung cancer, small cell lung cancer, colon cancer, and pancreatic cancer, promoting processes such as tumor cell proliferation, invasion, and metastasis. Its high expression is closely related to shortened survival and poor prognosis in cancer patients, making research on anti-tumor drugs targeting Trop2 of significant importance.
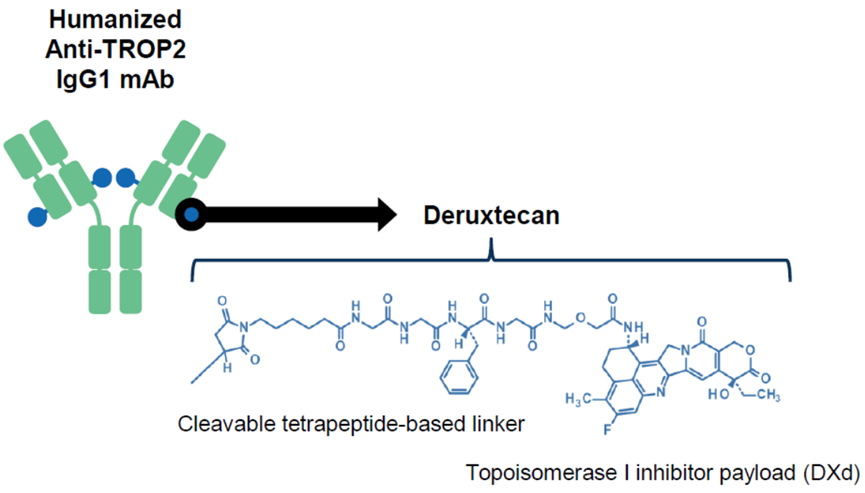 Figure 9: Schematic Structure of DS-1062
Figure 9: Schematic Structure of DS-1062
The fastest progressing Trop2-ADC drug is DS-1062, also developed by Daiichi Sankyo. DS-1062 links a humanized monoclonal antibody targeting the specific tumor cell surface antigen TROP2 with a novel topoisomerase 1 inhibitor exatecan derivative (DX-8951 derivative, DXd) through a 4-peptide linker. DXd is an innovative DNA topoisomerase I inhibitor with an activity ten times that of Irinotecan (SN-38), capable of interfering with DNA replication, recombination, and gene expression.
Currently, DS-1062 is undergoing a Phase III clinical trial comparing it to Docetaxel for the treatment of previously treated advanced/metastatic NSCLC patients. At the 2020 World Lung Cancer Conference (WCLC), AstraZeneca/Daiichi Sankyo disclosed preliminary clinical results from the Phase I clinical study TROPION-PanTumor01 of DS-1062 in treating advanced non-small cell lung cancer (NSCLC) patients. While confirming efficacy, its safety remains a significant limitation to its application. In the TROPION-PanTumor01 clinical trial, 14 patients (8%) experienced ILD, with most ILD cases (12/14) coming from the 8 mg/kg cohort, including 3 deaths (grade 5). In the 4 mg/kg dose group, there was 1 case of grade 3 ILD, and in the 6 mg/kg dose group, there was 1 case of grade 2 ILD. This raises the question of how to address the toxicity issues of ADC drugs, which is currently a hot topic in research.In addition to HER2, HER3, and TROP-2 targets, ADC drugs targeting TF (tissue factor) are also being applied clinically. Targeting Tissue Factor ADC
Targeting Tissue Factor ADC
On September 20, 2021, Seattle Genetics and Genmab A/S announced that their investigational Tisotumab vedotin received FDA accelerated approval for the treatment of recurrent or metastatic cervical cancer, becoming the first new drug for cervical cancer targeting TF, and the twelfth ADC drug approved globally.
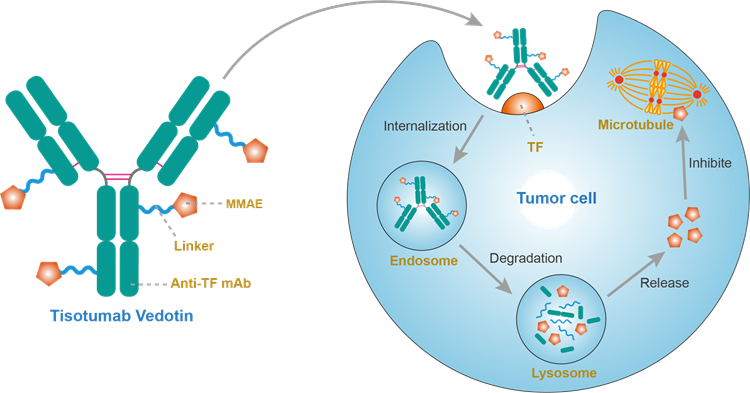 Figure 10: Mechanism of Action of Tisotumab vedotin
Figure 10: Mechanism of Action of Tisotumab vedotin
Tisotumab vedotin is a novel ADC drug that includes a monoclonal antibody targeting tissue factor (TF) and a microtubule-disrupting agent—Monomethyl auristatin E (MMAE). TF is abnormally expressed in various solid tumors, promoting tumor growth, neovascularization, and accelerating tumor metastasis. Tisotumab vedotin, upon binding to and internalizing with tumor cell surface TF, releases MMAE to induce cytotoxicity, effectively killing tumor cells, demonstrating excellent therapeutic effects in cervical cancer. It is currently being researched in multiple solid tumor areas, including NSCLC, with hopes of benefiting more patients.
 Conclusion
Conclusion
Although targeted therapies and immunotherapy-based strategies have become the first-line standard treatment for advanced lung cancer patients, in most cases, acquired resistance and disease progression remain inevitable. In such cases, chemotherapy is a common salvage option, but both toxicity and therapeutic effects are often unsatisfactory. The emergence of ADCs provides an attractive alternative. ADCs combine the specificity of monoclonal antibodies with the cytotoxic effects of chemotherapy to promote the direct targeting of cytotoxic payloads to cancer cells. However, while we focus on their efficacy, we must not relax our considerations regarding their toxicity. (🔗 ADC Drugs: Issues to be Resolved)
References
1.Reuss JE et al. Clinical Lung Cancer, Vol. 22, No. 6, 483–499.
2.Rosalin Mishra et al. Oncotarget, 2017, Vol. 8, (No. 69), pp: 114371-114392.
3.Mishra, Rosalin, et al. “HER3 signaling and targeted therapy in cancer.” Oncology reviews 12.1 (2018).
4.Mujoo, Kalpana, et al. “Regulation of ERBB3/HER3 signaling in cancer.” Oncotarget 5.21 (2014): 10222.
5.Yonemori K et al. Ann Oncol. 2019;30(suppl 3):iii47-iii64.
Editor: 💧Transparent
This post is intended to disseminate knowledge. If there are any copyright issues, please contact us within 30 days of publication.
Unauthorized reproduction of original content on other platforms is prohibited.
If you have questions, please email [email protected] for more information.
©2021 Medical Overview All rights reserved
Previous Links“The Journey of the Small Vaccine” | Pharmaceutical Company Pipeline Review Understanding Immunology | Understanding Immunology (Audio Version) Review Article Interpretation | Literature Briefs | Medical Popular Science | Pharmaceutical Frontline NotesPROTAC Technology | Antibody Drugs | Antibody-Drug Conjugates – ADCDNA Vaccines | CAR Technology | Chemical Biology

Warm Reminder
 The Medical Overview WeChat public account currently has nearly 7 discussion groups (knowledgeable, interesting, and bustling with talent in the pharmaceutical circle). To join, add the author on WeChat (yiyaosulan666) or scan the QR code of the public account, and note “Name/Nickname – Company/University – Specific Research Field/Major”. This group is strictly for research discussions, please do not disturb. A simple operation can star ⭐️ Medical Overview, to receive our updates promptly① Click on “Medical Overview” below the title ② Go to the top right corner “…” ③ Click “Set as Star”
The Medical Overview WeChat public account currently has nearly 7 discussion groups (knowledgeable, interesting, and bustling with talent in the pharmaceutical circle). To join, add the author on WeChat (yiyaosulan666) or scan the QR code of the public account, and note “Name/Nickname – Company/University – Specific Research Field/Major”. This group is strictly for research discussions, please do not disturb. A simple operation can star ⭐️ Medical Overview, to receive our updates promptly① Click on “Medical Overview” below the title ② Go to the top right corner “…” ③ Click “Set as Star”