Skip to content
History of ADC Drug Development
Phase 1 (1906-1982), the concept of targeted therapy was proposed, and the technology took shape.
Phase 2 (1983-2011), transition from clinical trials to commercialization, Mylotarg® was approved for market.
Phase 3 (2012-present), explosive growth of ADC products.
The five key elements of ADC drugs are target, antibody, linker, cytotoxic agent, and conjugation method.
-
Antibody selection determines the efficiency of Payload delivery and release, internalization efficiency, and lysosomal transport efficiency. ADC antibodies tend to have high target specificity, high affinity, low immunogenicity, high internalization, and long half-life, currently, the more mainstream subtype is IgG1.
-
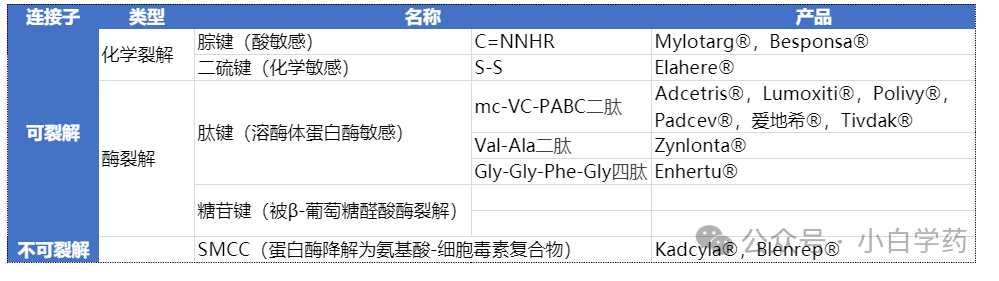
Linker, whose structure and cleavage properties are critical, cleavable linkers are the mainstream choice for ADCs, and currently, the most attention is on enzyme-cleavable linkers.
-
Payload, the type and toxicity of Payload need to be considered, with the main types currently being microtubule inhibitors and DNA damaging agents.
-
Conjugation technology employs site-specific conjugation to better control the DAR value.
Types of target antigens:
-
Overexpressed on tumor cell surfaces: EGFR, HER2, TROP-2, Nectin-4, etc.
-
Stromal antigens: Collagen IV (solid tumors), Periostin, Tenascin C
-
Vascular system antigens: PSMA, Tissue Factor
-
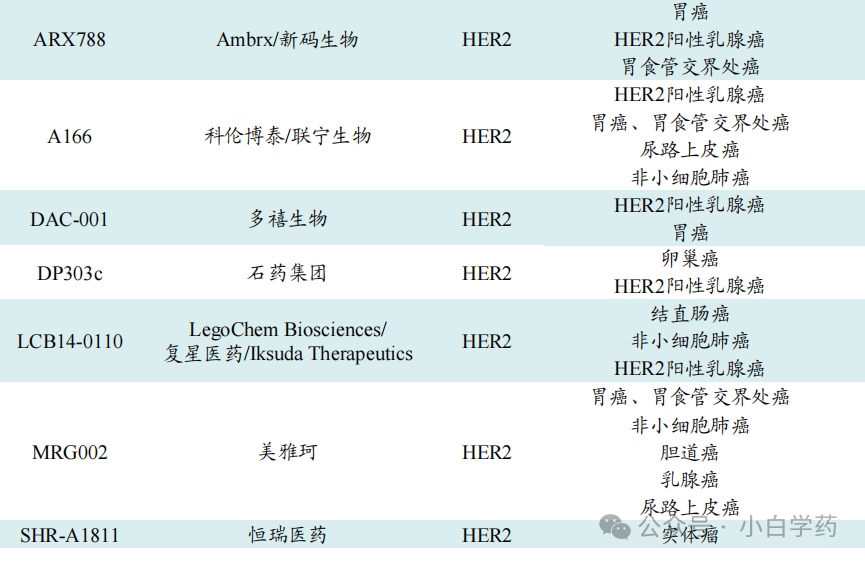
The competition for HER2 is intense, with pharmaceutical companies having ADC pipelines targeting this antigen including Kelun-Biotech, Yingensheng Biotechnology, Shiyao Zhongqi, Canbridge, Fosun, and Hengrui Medicine.
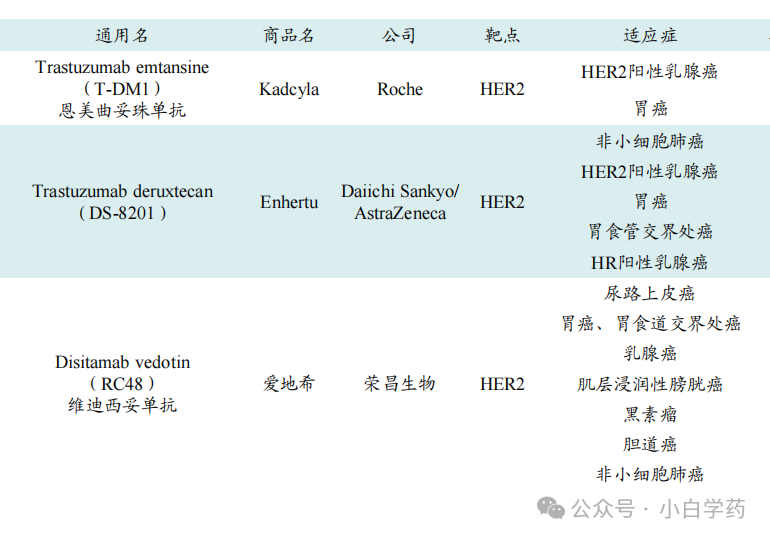 Table: Three HER2 ADC drugs already on the market
Table: Some HER2 ADC drugs under research in China
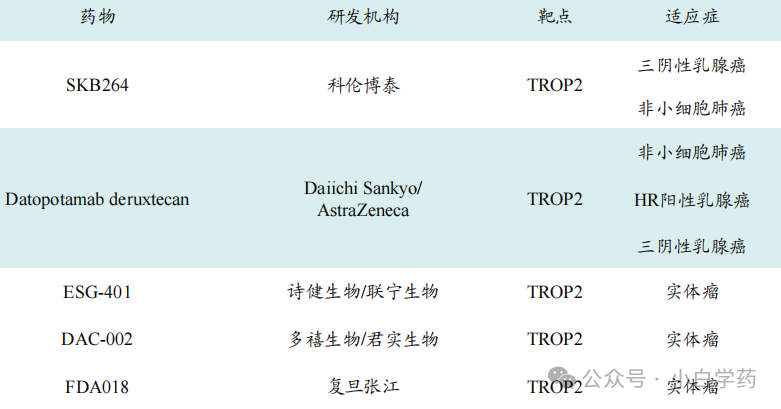
Next is TROP-2, with ADC companies targeting this antigen including Kelun-Biotech, Daiichi Sankyo, Gilead, etc.
Table: Some TROP-2 ADC drugs under research in China.
Other popular solid tumor targets also include: EGFR, CLDN18.2, Nectin-4, c-MET, etc.
Common targets in hematological malignancies include CD19, CD20, CD22, CD33, CD30, BCMA, CD79b.
The linker is a key structure that determines the efficacy and safety of ADCs. The linker releases toxins inside tumor cells while remaining stable in the bloodstream and non-target tissues.
Third-generation ADC drugs often use peptide linkers and adopt site-specific conjugation technology to improve the uniformity of drug-to-antibody ratio (DAR).
Peptide linkers can rapidly cleave under enzyme catalysis in lysosomes within cells, and do not easily break apart until they enter the tumor cells.
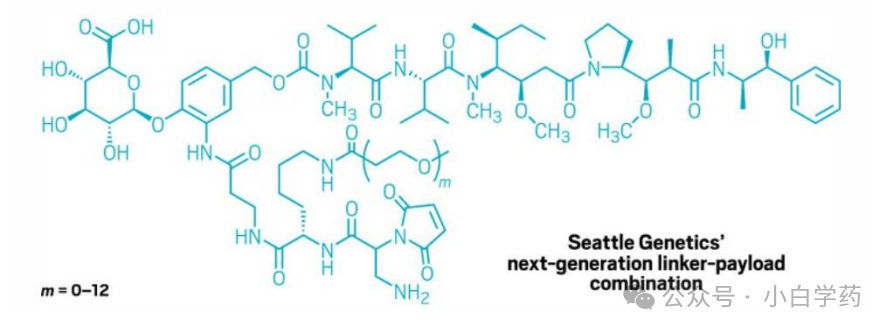
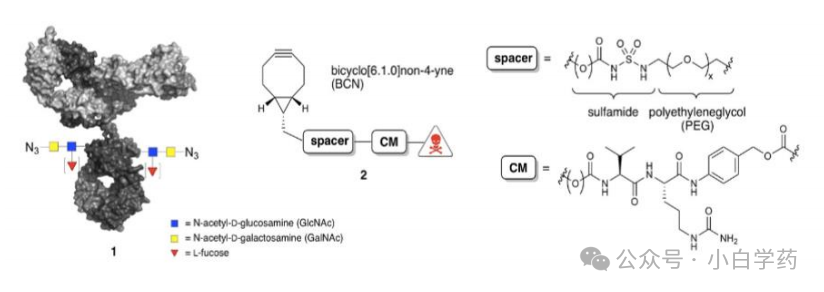
Seagen has developed hydrophilic linkers with PEG side chains. PEG designs can enhance system hydrophilicity, and different chain lengths (0-12) can modulate pharmacokinetics and non-specific uptake toxicity.
Figure: Seagen’s linker design
Synaffix employs a more hydrophilic design for linkers to improve overall ADC stability and increase Payload solubility. Synaffix increases solubility through higher polarity linkers.
Figure: Synaffix hydrophilic linker design
Effective Payloads mainly include microtubule inhibitors, DNA damaging agents, and immune modulators.
Effective payloads should have high anti-tumor activity, with single-agent IC50 reaching 0.01-0.1nM. Typically, only 2% of the drug reaches the tumor site after administration.
Table: Three HER2 ADC drugs already on the market
Table: Some HER2 ADC drugs under research in China
Next is TROP-2, with ADC companies targeting this antigen including Kelun-Biotech, Daiichi Sankyo, Gilead, etc.
Table: Some TROP-2 ADC drugs under research in China.
Other popular solid tumor targets also include: EGFR, CLDN18.2, Nectin-4, c-MET, etc.
Common targets in hematological malignancies include CD19, CD20, CD22, CD33, CD30, BCMA, CD79b.
The linker is a key structure that determines the efficacy and safety of ADCs. The linker releases toxins inside tumor cells while remaining stable in the bloodstream and non-target tissues.
Third-generation ADC drugs often use peptide linkers and adopt site-specific conjugation technology to improve the uniformity of drug-to-antibody ratio (DAR).
Peptide linkers can rapidly cleave under enzyme catalysis in lysosomes within cells, and do not easily break apart until they enter the tumor cells.
Seagen has developed hydrophilic linkers with PEG side chains. PEG designs can enhance system hydrophilicity, and different chain lengths (0-12) can modulate pharmacokinetics and non-specific uptake toxicity.
Figure: Seagen’s linker design
Synaffix employs a more hydrophilic design for linkers to improve overall ADC stability and increase Payload solubility. Synaffix increases solubility through higher polarity linkers.
Figure: Synaffix hydrophilic linker design
Effective Payloads mainly include microtubule inhibitors, DNA damaging agents, and immune modulators.
Effective payloads should have high anti-tumor activity, with single-agent IC50 reaching 0.01-0.1nM. Typically, only 2% of the drug reaches the tumor site after administration.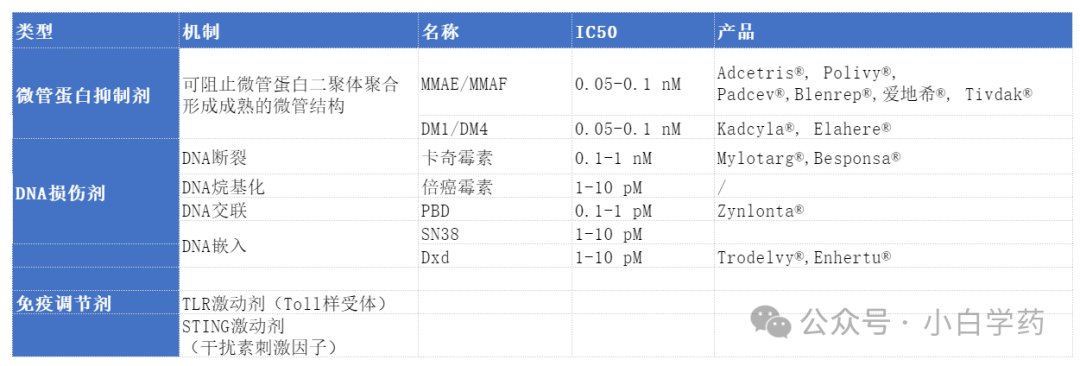 Microtubule inhibitors: can prevent microtubule protein dimers from polymerizing into mature microtubule structures. The most common are MMAE and MMAF, derivatives of auristatin, and DM1 and DM4, derivatives of maytansine.
The IC50 of DNA damaging agents can reach pM levels.
DNA crosslinking:N10/C11 imines can covalently crosslink with guanine’s N2 amino group, preventing DNA from binding with transcription factors, leading to cell proliferation stasis and ultimately cell death.
DNA embedding:Camptothecin derivatives can embed in the binding interface of TOP1 enzyme-DNA, preventing TOP1 from repairing DNA, resulting in DNA breaks.
TLR agonists:TLRs are a group of pattern recognition receptors in innate immunity, such as TLR7/8, which can activate the MyD88-dependent signaling pathway, further activating NF-κB to secrete cytokines and chemokines, causing infiltration of anti-tumor lymphocytes.
STING agonists:STING agonists bind to cGAMP, activating TBK1 kinase and IKK kinase inhibitors, further activating transcription factors IRF3 and NF-κB, inducing secretion of type I interferons and other inflammatory cytokines to kill tumors.
The first-generation ADC drugs mainly used chemotherapeutic agents, such as: Methotrexate, Vincristine, Doxorubicin, etc.
The second-generation ADC drugs mainly used microtubule inhibitors as Payloads, such as: DM1, DM4, MMAE, MMAF, etc.
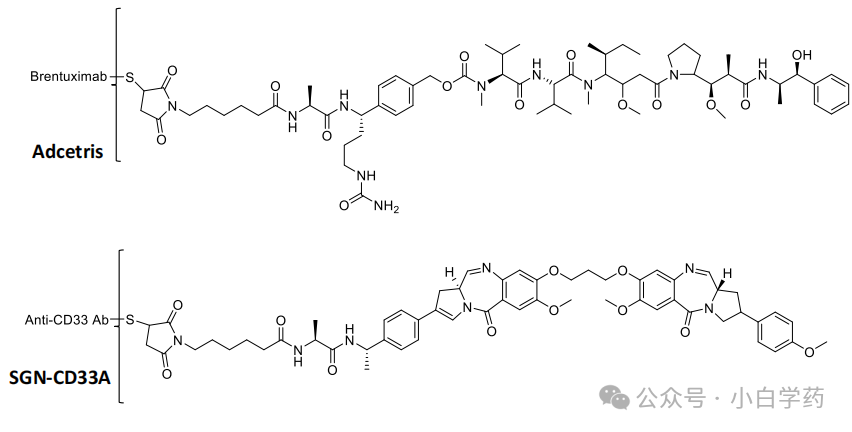
The third-generation ADC drugs Payload mainly use DNA damaging agents, such as: camptothecin derivatives, SN38, Dxd, etc. They destroy DNA structures through double-strand breaks, alkylation, embedding, and crosslinking, ultimately killing tumor cells.
Seagen’s early clinical designs were relatively conservative, previously using highly toxic Payload-PBD dimers. Seagen’s CD33-targeted ADC SGN-CD33A and CD70-targeted ADC SGN-CD70A both used PBD designs. Due to safety reasons, ADC development was halted in clinical trials, while new Payload-NAMPT inhibitors were being explored.
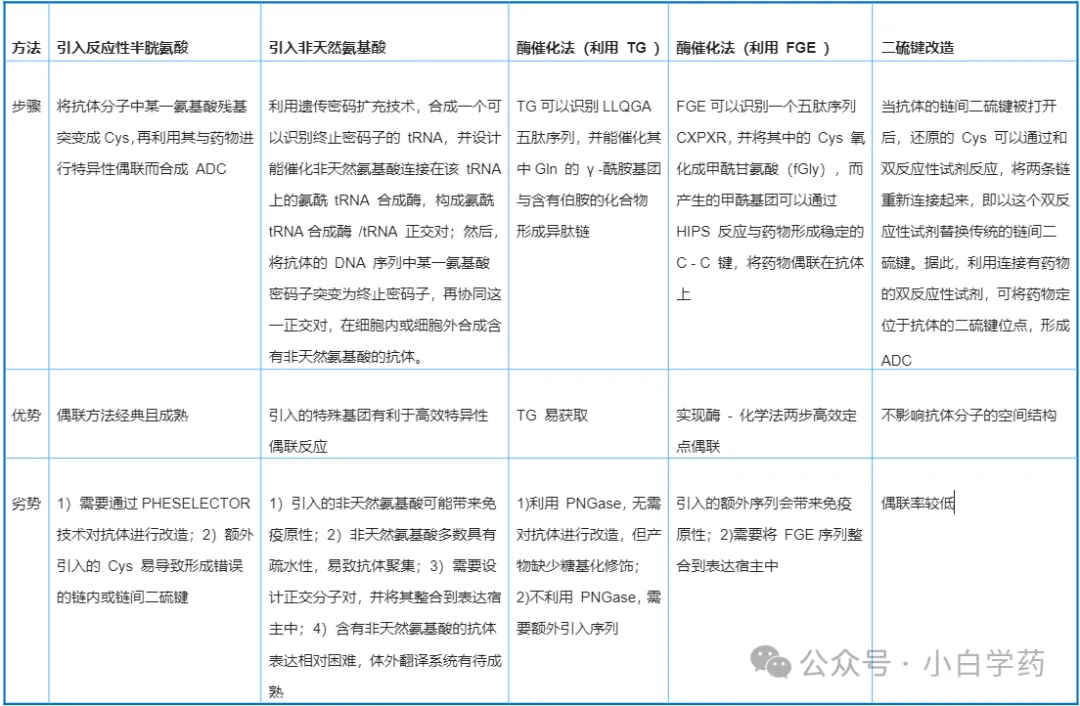
The conjugation method can affect the DAR value, thereby influencing drug uniformity, in vivo action processes, potency, pharmacological activity, tolerance, plasma clearance rate, pharmacokinetics, and drug stability. Conjugation methods can be divided into random conjugation and site-specific conjugation.
a) Lysine amide conjugation
In the early development of ADCs, lysine on the antibody was usually chosen as the binding site, which has a high synthesis efficiency.
However, since there can be up to 80 lysine residues on each antibody, this leads to significant heterogeneity, with an average DAR of 3.5-4, distributed between 0-7, affecting the in vivo distribution and cytotoxicity of ADC drugs.
Each antibody has 8 free cysteines, which can connect to the linker through disulfide bonds. Due to the limited number of binding sites and the unique reactivity of thiol groups, using cysteine as a binding site helps reduce ADC heterogeneity.
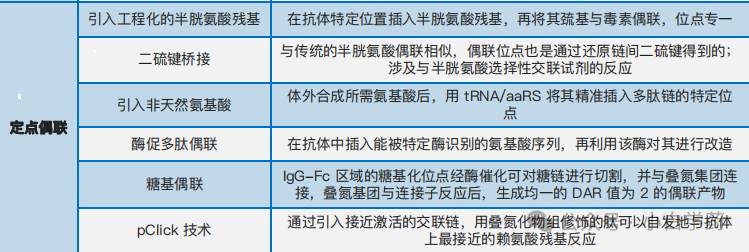
Site-Specific Conjugation
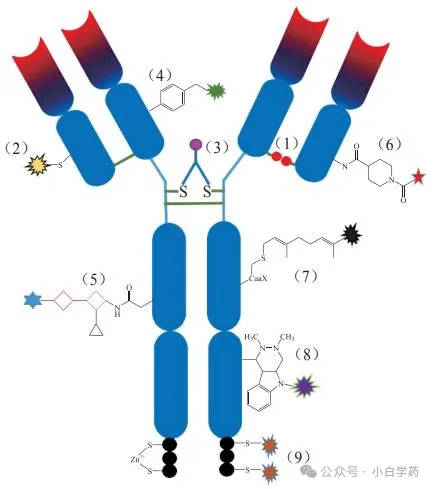
Cysteine conjugation technology (sites: 1, 2, 3)
Engineered non-natural amino acid conjugation technology (site: 4)
Glycosylation conjugation technology (site: 5)
Amino site-specific conjugation technology (site: 6)
Enzymatic conjugation technology (sites: 7, 8, 9)
Disulfide bridge conjugation: Open the disulfide bonds between antibody chains and use crosslinking reagents like bisulfone and maleimide to couple the effective payload to the thiol groups of cysteine, reconnecting the antibody.
Engineered reactive cysteine conjugation: The ThioMab platform developed by Genentech uses genetic engineering technology to insert cysteine residues at specific positions, coupling the payload to the thiol groups of cysteine, producing ADC drugs with a DAR value of 2.
Non-natural amino acid conjugation: Introduce non-natural amino acids to modify antibodies, allowing these special functional groups to achieve binding at specific sites.
Enzymatic conjugation: Through genetic engineering technology, artificially induce antibodies to express specific amino acid sequences, where amino acid residues in the sequence can be recognized and modified by enzymes, achieving site-specific conjugation. Commonly used enzymes include formylglycine-generating enzyme (FGE) and transglutaminase (TG).
Glycosylation conjugation: Use glycosidic endonucleases to expose the N-acetylglucosamine of the antibody Fc region, then use glycosyltransferases to connect it with azide-modified N-acetylgalactosamine, and finally connect the effective payload through click chemistry reactions.
Expanding targets and indications on mature platforms, continuously trying new toxins for Payloads, and hydrophilic designs for linkers, with site-specific conjugation technology being favored.
The success of T-DXd mainly includes:
Microtubule inhibitors: can prevent microtubule protein dimers from polymerizing into mature microtubule structures. The most common are MMAE and MMAF, derivatives of auristatin, and DM1 and DM4, derivatives of maytansine.
The IC50 of DNA damaging agents can reach pM levels.
DNA crosslinking:N10/C11 imines can covalently crosslink with guanine’s N2 amino group, preventing DNA from binding with transcription factors, leading to cell proliferation stasis and ultimately cell death.
DNA embedding:Camptothecin derivatives can embed in the binding interface of TOP1 enzyme-DNA, preventing TOP1 from repairing DNA, resulting in DNA breaks.
TLR agonists:TLRs are a group of pattern recognition receptors in innate immunity, such as TLR7/8, which can activate the MyD88-dependent signaling pathway, further activating NF-κB to secrete cytokines and chemokines, causing infiltration of anti-tumor lymphocytes.
STING agonists:STING agonists bind to cGAMP, activating TBK1 kinase and IKK kinase inhibitors, further activating transcription factors IRF3 and NF-κB, inducing secretion of type I interferons and other inflammatory cytokines to kill tumors.
The first-generation ADC drugs mainly used chemotherapeutic agents, such as: Methotrexate, Vincristine, Doxorubicin, etc.
The second-generation ADC drugs mainly used microtubule inhibitors as Payloads, such as: DM1, DM4, MMAE, MMAF, etc.
The third-generation ADC drugs Payload mainly use DNA damaging agents, such as: camptothecin derivatives, SN38, Dxd, etc. They destroy DNA structures through double-strand breaks, alkylation, embedding, and crosslinking, ultimately killing tumor cells.
Seagen’s early clinical designs were relatively conservative, previously using highly toxic Payload-PBD dimers. Seagen’s CD33-targeted ADC SGN-CD33A and CD70-targeted ADC SGN-CD70A both used PBD designs. Due to safety reasons, ADC development was halted in clinical trials, while new Payload-NAMPT inhibitors were being explored.
The conjugation method can affect the DAR value, thereby influencing drug uniformity, in vivo action processes, potency, pharmacological activity, tolerance, plasma clearance rate, pharmacokinetics, and drug stability. Conjugation methods can be divided into random conjugation and site-specific conjugation.
a) Lysine amide conjugation
In the early development of ADCs, lysine on the antibody was usually chosen as the binding site, which has a high synthesis efficiency.
However, since there can be up to 80 lysine residues on each antibody, this leads to significant heterogeneity, with an average DAR of 3.5-4, distributed between 0-7, affecting the in vivo distribution and cytotoxicity of ADC drugs.
Each antibody has 8 free cysteines, which can connect to the linker through disulfide bonds. Due to the limited number of binding sites and the unique reactivity of thiol groups, using cysteine as a binding site helps reduce ADC heterogeneity.
Site-Specific Conjugation
Cysteine conjugation technology (sites: 1, 2, 3)
Engineered non-natural amino acid conjugation technology (site: 4)
Glycosylation conjugation technology (site: 5)
Amino site-specific conjugation technology (site: 6)
Enzymatic conjugation technology (sites: 7, 8, 9)
Disulfide bridge conjugation: Open the disulfide bonds between antibody chains and use crosslinking reagents like bisulfone and maleimide to couple the effective payload to the thiol groups of cysteine, reconnecting the antibody.
Engineered reactive cysteine conjugation: The ThioMab platform developed by Genentech uses genetic engineering technology to insert cysteine residues at specific positions, coupling the payload to the thiol groups of cysteine, producing ADC drugs with a DAR value of 2.
Non-natural amino acid conjugation: Introduce non-natural amino acids to modify antibodies, allowing these special functional groups to achieve binding at specific sites.
Enzymatic conjugation: Through genetic engineering technology, artificially induce antibodies to express specific amino acid sequences, where amino acid residues in the sequence can be recognized and modified by enzymes, achieving site-specific conjugation. Commonly used enzymes include formylglycine-generating enzyme (FGE) and transglutaminase (TG).
Glycosylation conjugation: Use glycosidic endonucleases to expose the N-acetylglucosamine of the antibody Fc region, then use glycosyltransferases to connect it with azide-modified N-acetylgalactosamine, and finally connect the effective payload through click chemistry reactions.
Expanding targets and indications on mature platforms, continuously trying new toxins for Payloads, and hydrophilic designs for linkers, with site-specific conjugation technology being favored.
The success of T-DXd mainly includes:
-
Breast cancer indications defeating T-DM1;
-
Expanding application range to HER2 low expression;
-
Daiichi Sankyo’s second-generation ADC technology design includes:
-
Payload using highly toxic PBD dimers;
-
Antibody connection points modified by glycosylation and coupled to linkers through “click reactions”;
-
Linkers using the peptide sequence GGVA;
-
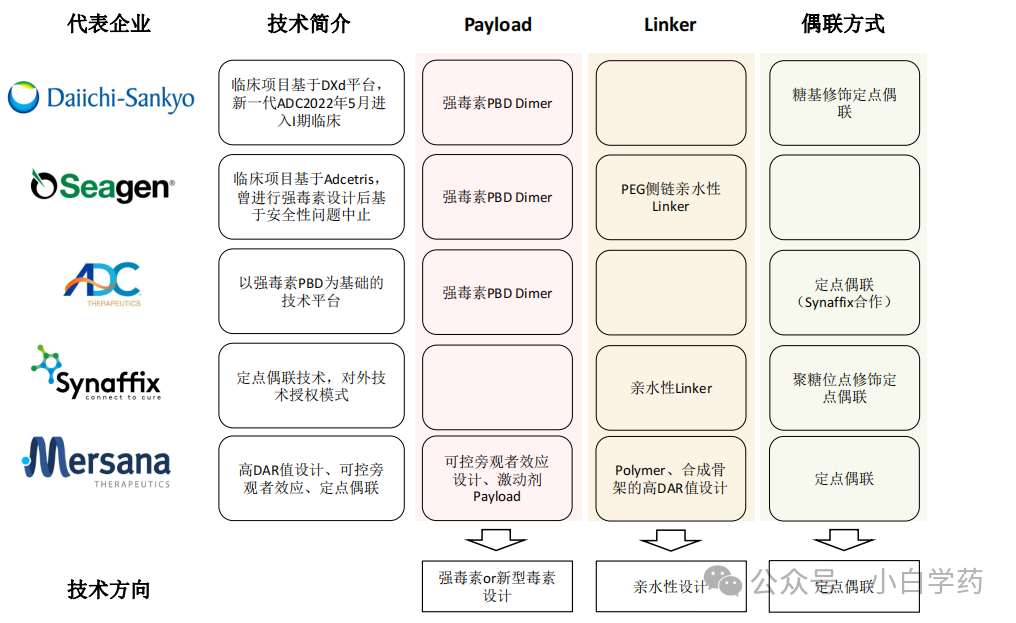
Figure: Technical development directions of overseas ADC companies
Domestic ADC companies can be divided into: 1. Companies that have established technical platforms and are expanding targets on these platforms, focusing on clinical progress of projects. 2. Companies with relatively unique designs and projects with potential to become FIC or BIC.
Target antigens: expanding from tumor cell surface antigens to targets in the tumor microenvironment.
Linkers: 1) Cleavable linkers are the mainstream direction. 2) Improve hydrophilicity to increase the number of effective payloads on a single linker.
Effective Payloads: 1) DNA topoisomerase I inhibitors as representatives of new generation toxic molecules, effective against non-dividing cancer cells and can kill cancer cells resistant to microtubule inhibitors. 2) ADP/elongation factor 2 inhibitors and near-infrared photosensitizers have already been marketed. 3) RNA polymerase II inhibitors.
Conjugation technology: Site-specific conjugation technology achieves drug loading at specific sites through antibody modification, resulting in ADC drugs with high uniformity and good safety, thereby widening the therapeutic window. This will become an important development direction in the future.
Scan the WeChat QR code to add the Antibody Circle editor, qualified individuals can join the Antibody Circle WeChat group!
Please indicate: Name + Research Direction!
All articles reprinted by this public account are for the purpose of conveying more information, and sources and authors are clearly marked. Media or individuals who do not wish to be reprinted can contact us ([email protected]), and we will immediately delete it. All articles only represent the author’s views and do not represent the position of this site.

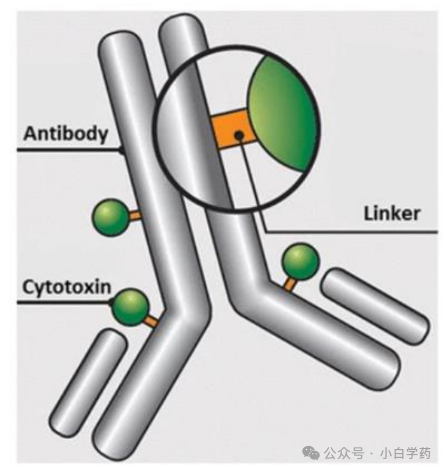
 Table: Three HER2 ADC drugs already on the market
Table: Three HER2 ADC drugs already on the market