
Please click the “Bio New Thinking” above to follow more exciting content!
Written by / Jia Bei Pharmaceutical @ Bai Ao Yun Testing
Reviewed by / Gu Du Pharmacist @ Bio New Thinking
Antibody-drug conjugates (ADCs) are large molecule drugs formed by conjugating monoclonal antibodies with highly potent small molecule drugs via linkers, enabling targeted effects of small molecules at the site of cancer cells. Due to their outstanding efficacy, they have become one of the fastest-growing cancer treatment methods. According to Grandview statistics, with various ADC drugs being launched and their applications expanding to more diseases, the industry scale of ADCs will grow rapidly in the future.
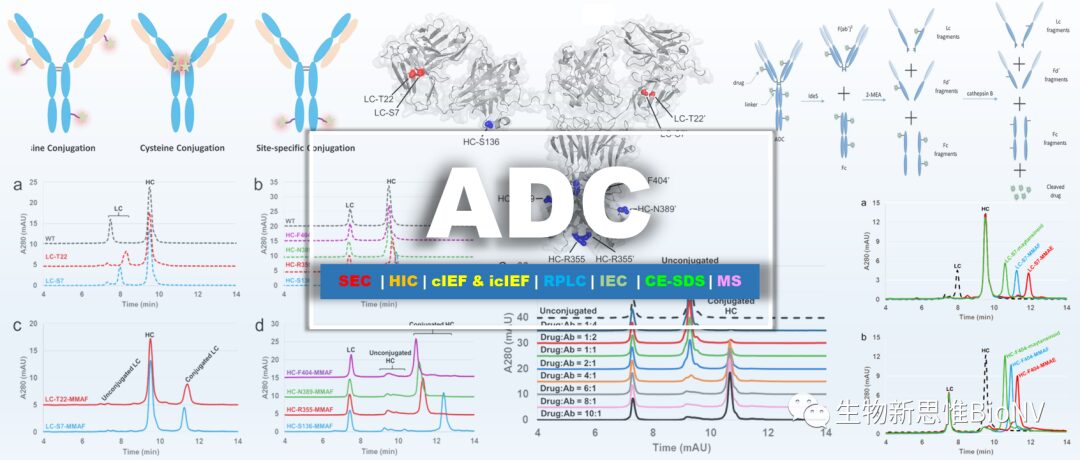
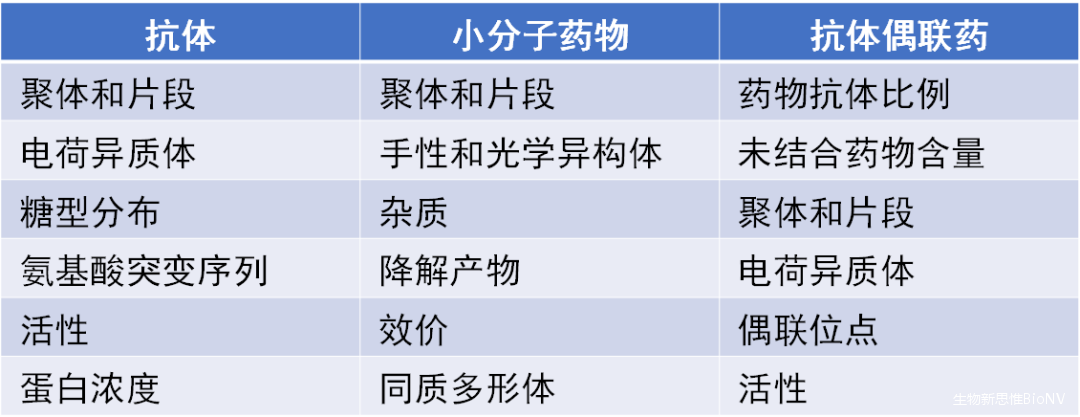
Generally, the physicochemical analysis and structural characterization of ADCs include the detection and analysis of characteristic parameters of individual antibodies, small molecule drugs, and linkers, as well as the detection of ADC-specific properties such as the drug-antibody ratio (DAR), drug distribution, unconjugated antibody ratio, and conjugation sites. In light of this, this article comprehensively introduces the technical methods related to the physicochemical analysis and structural characterization of ADCs.
Table 1 Key Content of Structural Analysis and Quality Control of ADC Drugs

Size exclusion chromatography (SEC) is a technique that separates proteins based on differences in molecular size, which can be used to analyze the molecular weight and apparent molecular weight of ADCs, and is the most commonly used method for studying molecular size variants.
When analyzing with SEC, a high ionic strength aqueous solution is typically required as the mobile phase to shield the interactions between the molecules and the stationary phase. However, since the small molecules conjugated to ADCs are usually highly hydrophobic, continuing to use the mobile phase for monoclonal antibody SEC analysis may lead to issues such as wider SEC result peaks and poorer resolution.
Therefore, by adding a small amount of organic solvent to the mobile phase, SEC results can be improved, for example: acetonitrile, isopropanol, etc. (Figure 1). Additionally, the type of ions in the mobile phase may significantly affect the separation effect of SEC for ADCs (Figure 2).
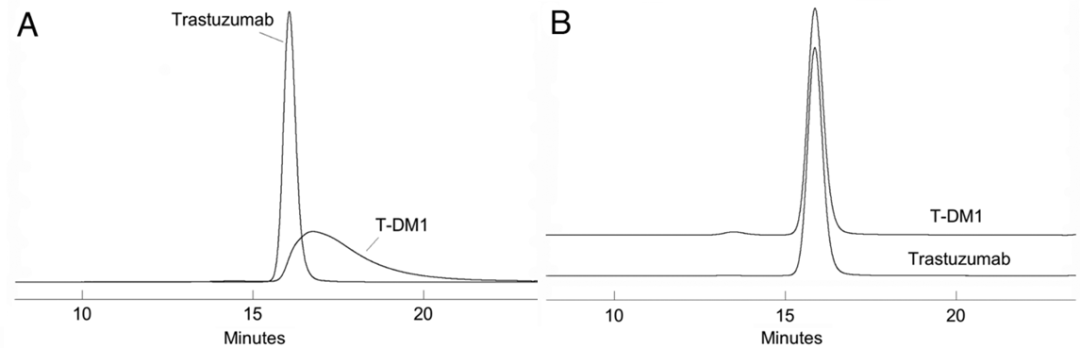
Figure 1 SEC Chromatograms of mAb (Trastuzumab) and ADC (T-DM1)
(A: No organic solvent; B: 15% isopropanol added)
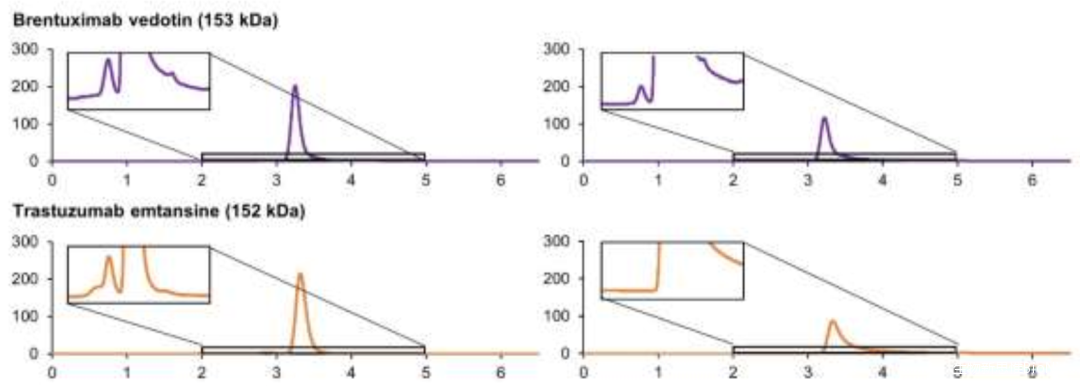
Figure 2 SEC Chromatograms of Two ADCs(Left: Potassium Phosphate; Right: Ammonium Acetate)

Hydrophobic interaction chromatography (HIC) separates based on the strength of hydrophobic interactions between different protein molecules and the stationary phase, maintaining the natural conformation of protein samples during the separation process. It is a typical efficient, non-denaturing analytical method that can be used to detect and calculate the DAR value and the ratio of unconjugated antibody molecules in ADCs.
For HIC, both the properties of the mobile phase and the stationary phase significantly influence its separation effectiveness. Factors related to the mobile phase include whether to add organic solvents, which organic solvent to add, and the concentration gradient of the organic solvent chosen. Additionally, the nature of the stationary phase is also crucial.
Taking the marketed ADC drug Kadcyla as an example, the amount of organic solvent added during the HIC separation process significantly affects the peak time and peak shape of ADC molecules with different DAR values, and it also influences the final DAR value calculation results. As shown in Figure 3, when the organic solvent isopropanol (or acetonitrile) content in mobile phase B is 18%, it can effectively distinguish ADC molecules with different DAR values. Moreover, compared to the commonly used linear gradient, setting the gradient of the mobile phase to a logarithmic gradient during separation can yield better separation. This is because when the number of small molecule drugs is low, the increase in the number of small molecule drug conjugates has a significant impact on the hydrophobicity of the ADC drug, while the hydrophobicity difference between two different ADCs with DAR values of 6 and 8 is not significant, thus requiring a smaller organic solvent concentration gradient to achieve better separation (Figure 4).
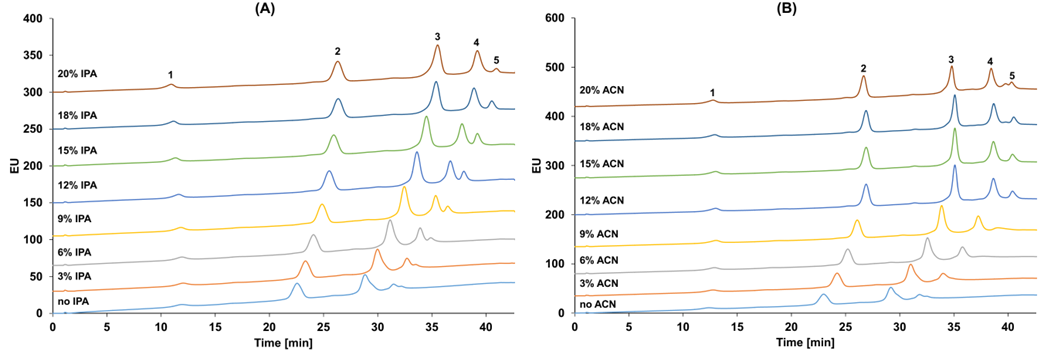
Figure 3 HIC Results of Kadcyla(1: DAR0; 2: DAR2; 3: DAR4; 4: DAR6; 5: DAR8). Separation conditions: Mobile Phase A: 4.5 M NH4OAc; Mobile Phase B: 20 mM NH4OAc containing different concentrations of IPA (A) and ACN (B).
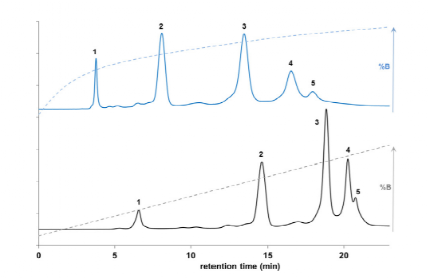
Figure 4 Comparison of Logarithmic and Linear Gradient HIC Separation Results for KadcylaMobile Phase A: 4 M NaCl and 10 nM phosphate buffer; Mobile Phase B: 10 mM phosphate buffer and 8% isopropanol.

RPLC is similar to HIC; both aim to separate based on the hydrophobic interactions between protein molecules and the stationary phase.
The difference is that RPLC’s stationary phase typically has stronger hydrophobicity; when analyzing ADCs with HIC, no sample pre-treatment is required, whereas RPLC analysis usually requires the reduction of ADC molecules with TECP or DTT for higher resolution, and thus analyzes key quality attributes (CQA) such as DAR value and conjugation sites.
In the analysis of ADC molecules designed with Cys or site-specific conjugation strategies, RPLC is widely used. As shown in Figure 5, using RPLC to analyze several ADCs with site-specific conjugation, their conjugation sites are LC-S7 (a) and HC-F404 (b), and calculations show their DAR value is 2. For ADC drugs that adopt random Cys conjugation, the specific sites of cysteine conjugation can typically be analyzed by comparing RPLC results with those of SDS capillary electrophoresis (CE-SDS).
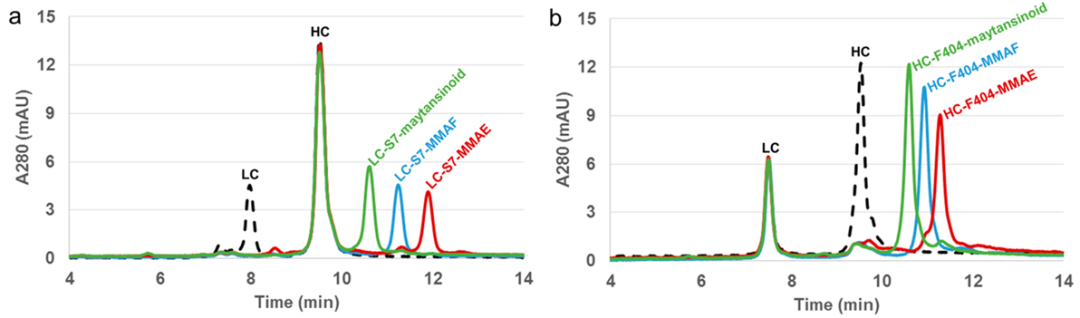
Figure 5 RPLC Results of Conjugation Sites in ADC Drugs at LC-S7 (a) and HC-F404 (b)

Ion exchange chromatography (IEC) separates based on the differences in binding capacity of different samples with the stationary phase at different pH mobile phases, which is a typical non-denaturing, charge heterogeneity analysis method, where salt ions and pH gradients significantly affect separation results.
IEC is suitable for analyzing ADC drugs where small molecule conjugation significantly affects antibody charge, such as: lysine conjugation; linkers and small molecules carrying charges, etc.

Sodium Dodecyl Sulfate Capillary Electrophoresis (CE-SDS)
Sodium dodecyl sulfate capillary electrophoresis (CE-SDS) separates protein samples based on the molecular sieve effect and is an efficient tool for studying the size heterogeneity of ADCs. In this method, since SDS can disrupt inter-chain hydrogen bonds, it is more suitable than SEC as a routine analysis method for protein fragments.
When analyzing ADC drugs using CE-SDS, method development should be based on the conjugation method and the type of small molecule drugs, specifically:
Cys Conjugated ADC: Typically compares CE-SDS results with HIC, RPLC, etc. to obtain CQA information (Figure 6)/
Lys Conjugated ADC: Can be analyzed by comparing non-reducing CE-SDS and reducing CE-SDS results. It is worth noting that this method is more suitable for ADCs with relatively large molecular weights of small molecule drugs. Moreover, compared to non-reducing CE-SDS, the reducing CE-SDS spectra have higher discrimination for different DAR value molecules (Figure 7)/
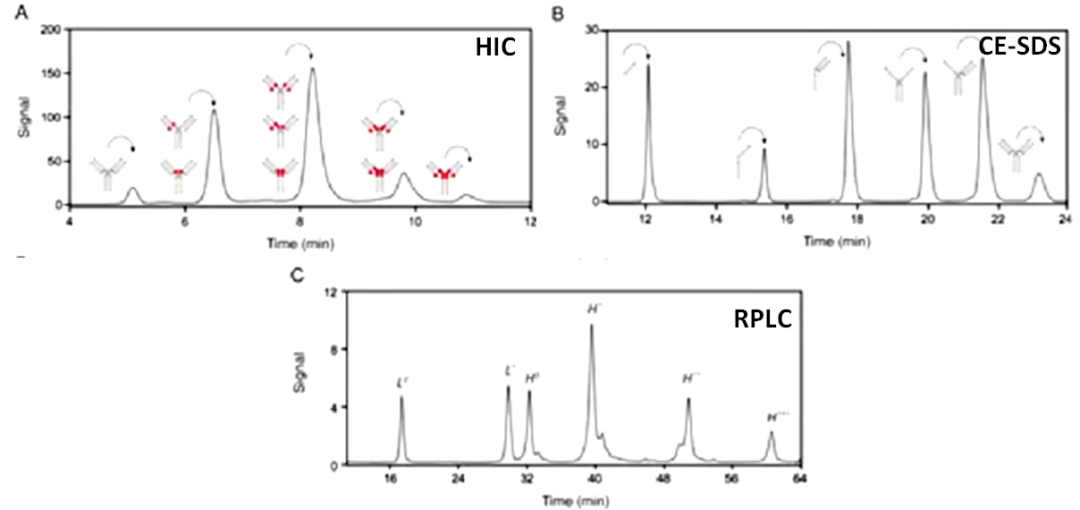
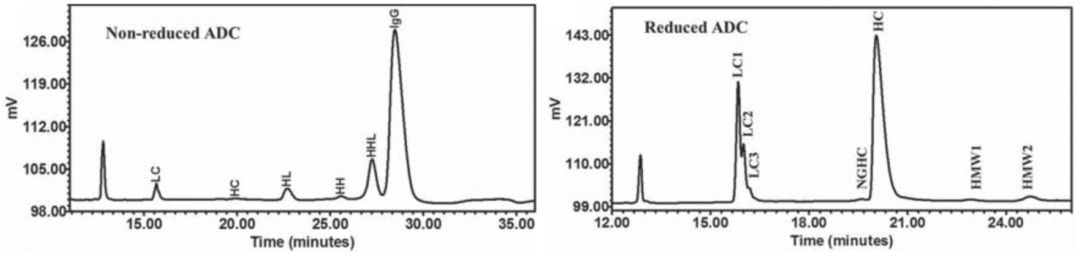
Figure 7 CE-SDS Results of Lys Conjugated ADC Drugs

Capillary Isoelectric Focusing / Imaging Capillary Isoelectric Focusing Electrophoresis (cIEF/icIEF)
Capillary isoelectric focusing/imaging capillary isoelectric focusing electrophoresis (cIEF/icIEF) is a method that separates samples based on the isoelectric point (pI) differences of protein samples, making it an effective tool for studying the charge heterogeneity of ADCs.
Similar to IEF, cIEF/icIEF is also suitable for analyzing ADCs where charge changes occur after conjugation. However, cIEF has higher resolution than IEF, providing characteristic molecular profiles of ADCs, and thus can be used for product release testing (Figure 8).
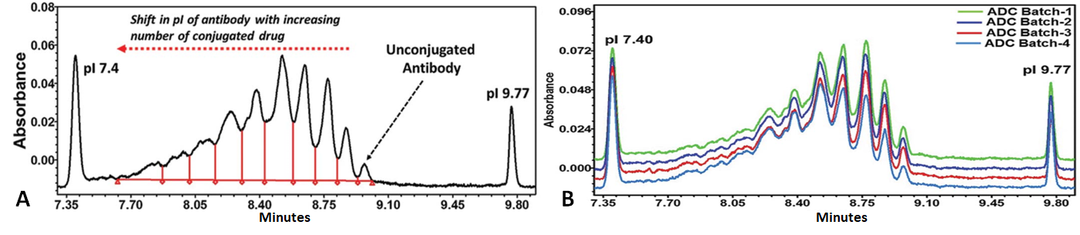
Figure 8 cIEF Results of Lys Conjugated ADC Drugs(A, Fingerprint Profile; B, cIEF Profiles of Different Batches of ADC Drugs)

Mass spectrometry-based analysis methods can analyze the characteristics of ADCs from various scales, including intact molecules, subunits, and peptide segments, making it the best tool for CQA analysis and consistency evaluation of ADC drugs.
For different conjugation methods, appropriate MS analysis methods should be developed to achieve better results. For example, combining RPLC, SEC-HPLC, or capillary electrophoresis with mass spectrometry can be used to analyze and characterize ADC features such as drug-antibody ratio (DAR), drug distribution, unconjugated antibody ratio, and conjugation sites (Figures 9-11 and Table 2).
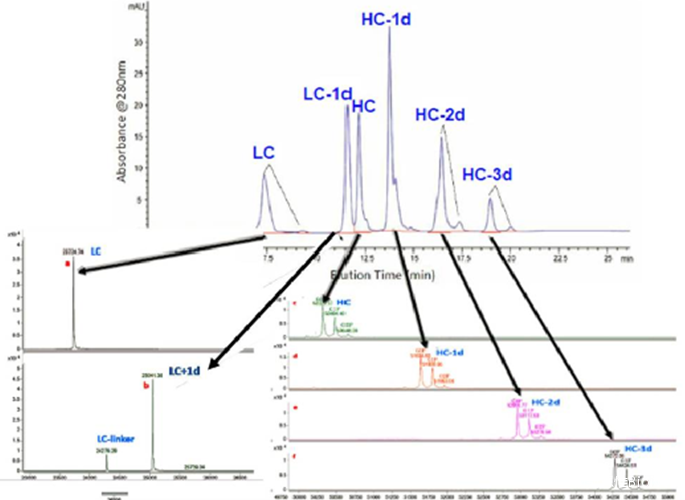
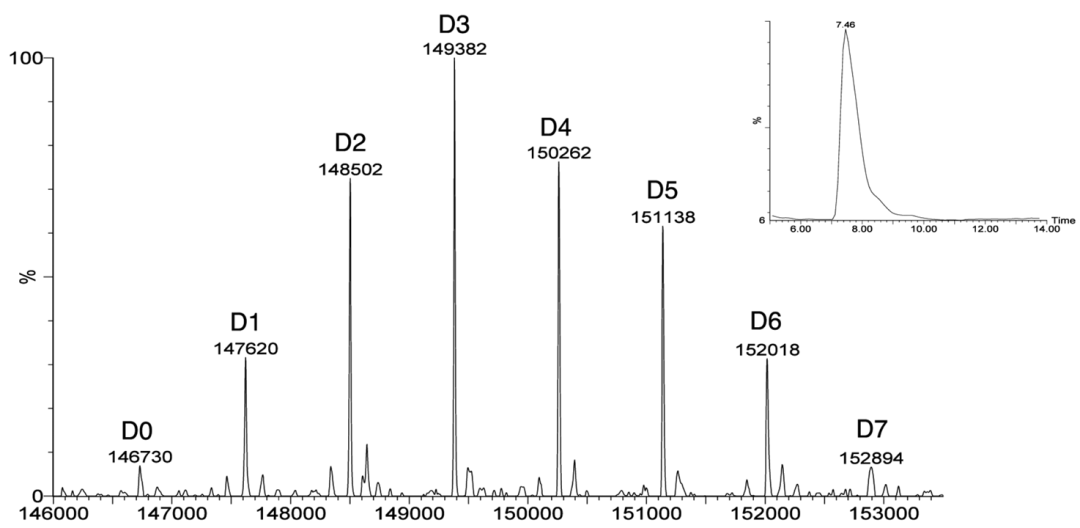
Figure 10 Analyzing the DAR and Drug Distribution of ADCs Using SEC-MS
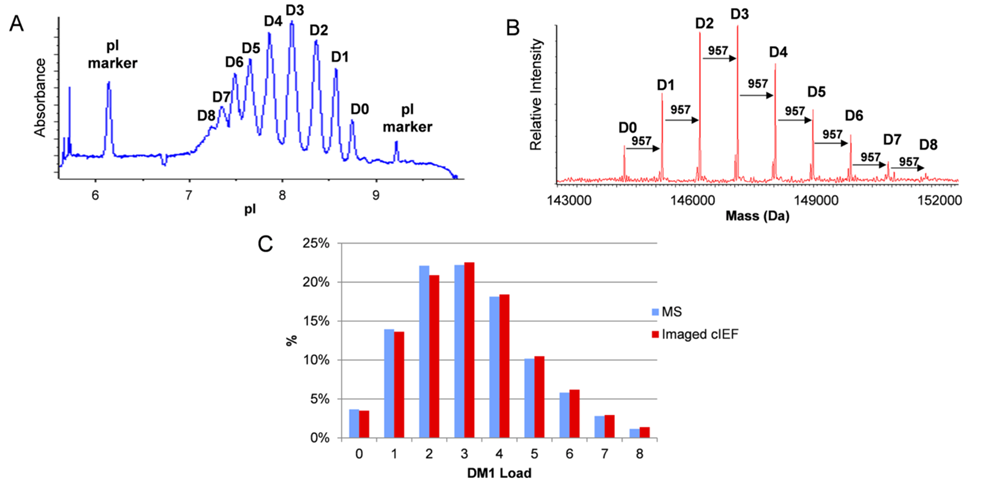
Figure 11 Analyzing the Drug Distribution of ADCs by Comparing cIEF with MS Results
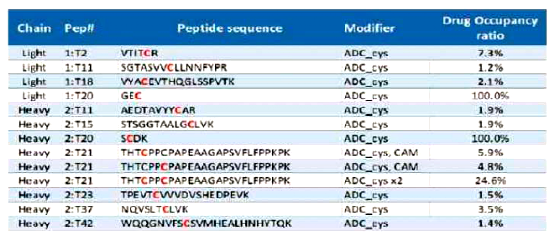
Table 2 Characterizing the Conjugation Sites and Ratios of ADCs Using MS Methods

In the past few decades, significant progress has been made in research on ADC drugs in various fields such as conjugation chemistry, production, and product stability.
Compared to naked antibodies, the inherent complexity of ADC drug molecules poses significant challenges for the development of analytical methods. To better control the quality, stability, and safety of ADC drugs, it is crucial to have a more comprehensive and in-depth understanding of the characteristics of ADC drug molecules. Therefore, it is necessary to choose appropriate tools for multidimensional detection and analysis of ADC molecules based on conjugation methods and types of small molecule drugs.
Written by / Jia Bei Pharmaceutical @ Bai Ao Yun Testing
Reviewed by / Gu Du Pharmacist @ Bio New Thinking
Previous Recommendations
Upstream Technology | Simplifying the CRISPR Editing Process to Improve iPSC Clonal Efficiency
Upstream Technology | Six Major Host Expression Systems for Therapeutic Proteins
Upstream Technology | Screening Strategies for Stable High-Yield CHO Cells
Upstream Technology | The Mysterious Post-Translational Modifications of Therapeutic Proteins—Tyrosine Sulfation and Its Regulation Strategies
Upstream Technology | Protein Hydrolysates and Their Applications in Biopharmaceutical Production
Upstream Technology | CMC Cornerstone—Cell Line Development Process in Biologics Production
Upstream Technology | The Path to Industrialization of Adherent Cells
Upstream Technology | Bispecific Antibodies Are Prone to Aggregation; Continuous Processes Solve the Problem
Upstream Technology | Key Influencing Factors of Monoclonal Antibody Glycosylation Modifications and Their Regulation Strategies
Upstream Technology | New Hope in the Post-COVID Era: HEK293 Cells and Their Key Upstream Technologies
Upstream Technology | Diversified Continuous Flow Processes Drive Cost Changes in Biopharmaceuticals
Upstream Technology | Detailed Explanation of the Reduction and Fragmentation Issues of Bispecific Antibodies
Upstream Technology | Continuous Flow Processes Mitigate the Intracellular Mechanisms of Bispecific Antibody Aggregation
Upstream Technology | Core Technologies and Key Steps in Recombinant Protein Production Based on Cell Culture
Upstream Technology | How to Break Down Lactic Acid Accumulation? Detailed Explanation of the Driving Mechanisms and Regulation Strategies of Lactic Acid Consumption in CHO Cells
Upstream Technology | A Comprehensive Overview of the Challenges and Countermeasures in Regulating High Mannose Modification of Monoclonal Antibodies
Upstream Technology | Deep Understanding of the Mechanisms of pH Regulation on Monoclonal Antibody PQA
Upstream Technology | Better Performance in Large-Scale Processes? Perhaps Due to High Hydrostatic Pressure
Upstream Technology | Is “Aggregation” the Fate of “High Expressers”? How to Resolve the Conflict Between Protein Aggregation and Secretion Efficiency?
Upstream and Downstream Technology | Process Insight: PAT and Digital Twins in Continuous Biologics Production
Upstream and Downstream Technology | How to Regulate Acid/Base Heterogeneity? Overview of the Sources, Influences, Analyses, and Regulation Strategies of Charge Heterogeneity
Downstream Technology | Learning from Others: Purification Strategies for Bispecific Antibodies Based on Mixed-Mode Chromatography
Downstream Technology | Differentiating Affinity: Purification Techniques Based on Hydrophobic Interactions in Bispecific Antibody Production
Downstream Technology | Size Matters: Purification Techniques Based on Molecular Size for Bispecific Antibodies
Downstream Technology | Charge-Based Purification Techniques for Bispecific Antibodies
Downstream Technology | “Four Great Detectives”: Affinity Chromatography Capture Techniques for Bispecific Antibodies
Downstream Technology | Comprehensive Analysis of the Reasons for and Solutions to Disulfide Bond Reduction in Antibodies
Downstream Technology | Research Progress on Monoclonal Antibody Purification Processes and Their Commercial Production
Characterization Techniques | Efficient Characterization Strategies for HCP in Therapeutic Protein Production
Characterization Techniques | Instability of Fc Fusion Proteins and Research Strategies for Forced Degradation
Characterization Techniques | Molecular Size Heterogeneity of Fc Fusion Proteins and Its Characterization Strategies
Characterization Techniques | Glycosylation Modifications of Fc Fusion Protein Molecules and Their Characterization Strategies
Characterization Techniques | Primary Structure Characterization Techniques for Therapeutic Fc Fusion Proteins
Characterization Techniques | Charge Heterogeneity Characterization of Therapeutic Fc Fusion Proteins
Characterization Techniques | Primary Structure Characterization Techniques for Therapeutic Fc Fusion Proteins
Characterization Techniques | Detailed Explanation of New Techniques for Characterizing Monoclonal Antibody Glycosylation Modifications
Formulation Techniques | The Stabilizing Effect of Polysorbate on Biologics
Formulation Techniques | General Process and Key Considerations for Biopharmaceutical Formulation Development
Formulation Techniques | Challenges and Innovative Solutions in Biologic Container Development
Cell Therapy | Regulating NK Cell Metabolism to Enhance Tumor Killing Efficiency
Special Detailed Explanation | Large-Scale Production Processes of Therapeutic Recombinant Enzyme Preparations
Special Detailed Explanation | Metabolic Regulation of Immune Cells in Tumor Microenvironment
Special Detailed Explanation | Limitations of CAR-T Cell Therapy and Improvement Strategies
Special Detailed Explanation | New Bottles for Old Wine: Challenges and Countermeasures in the Iterative Upgrading of Biopharmaceutical Old Processes
Special Detailed Explanation | The Cornerstone and Bottlenecks of mRNA Technology Platforms
Special Detailed Explanation | Fc-Fusion: Therapeutic Proteins Based on Antibody Fc Fragments
Special Detailed Explanation | Major Challenges and Response Strategies in GMP Production and Quality Supervision of Biologics
Special Detailed Explanation | Quality Management in the Development Process of Biologics
Special Detailed Explanation | Principles of Buffer System Selection and Vendor Management in Biologics Production
Special Detailed Explanation | Metabolic Mechanisms of Tumor Evasion from NK Cell “Chasing”
Health Thinking | Counteracting Aging: Can Caloric Control Delay Aging?
Industry Dynamics | New Breakthroughs in COVID Drugs: Professor Lu Bai and Doctor Dou Yang from Tsinghua University School of Pharmacy Publish Series of Articles Reporting COVID Drug Development Achievements
Industry Dynamics | General Process and Key Considerations in the Development of Antibody-Drug Conjugates (ADCs)
Industry Dynamics | Who Will Disrupt the Future of Innovative Drugs in the Next 20 Years?
Industry Dynamics | Leading Companies and Their Pipelines in the mRNA Vaccine Field
1. Wagh, A.; Song, H.; Zeng, M.; Tao, L.; Das, T. K., Challenges and new frontiers in analytical characterization of antibody-drug conjugates. mAbs 2018,10 (2), 222-243.
2. Xu, Y.; Jiang, G.; Tran, C.; Li, X.; Heibeck, T. H.; Masikat, M. R.; Cai, Q.; Steiner, A. R.; Sato, A. K.; Hallam, T. J.; Yin, G., RP-HPLC DAR Characterization of Site-Specific Antibody Drug Conjugates Produced in a Cell-Free Expression System. Organic Process Research & Development 2016,20 (6), 1034-1043.
3. Wiggins, B.; Liu-Shin, L.; Yamaguchi, H.; Ratnaswamy, G., Characterization of Cysteine-Linked Conjugation Profiles of Immunoglobulin G1 and Immunoglobulin G2 Antibody–Drug Conjugates. Journal of Pharmaceutical Sciences 2015,104 (4), 1362-1372.
4. Luo, Q.; Chung, H. H.; Borths, C.; Janson, M.; Wen, J.; Joubert, M. K.; Wypych, J., Structural Characterization of a Monoclonal Antibody–Maytansinoid Immunoconjugate. Analytical Chemistry 2016,88 (1), 695-702.
5. Rodriguez-Aller, M.; Guillarme, D.; Beck, A.; Fekete, S., Practical method development for the separation of monoclonal antibodies and antibody-drug-conjugate species in hydrophobic interaction chromatography, part 1: optimization of the mobile phase. Journal of Pharmaceutical and Biomedical Analysis 2016,118, 393-403.
6. Bobaly, B.; Beck, A.; Veuthey, J.-L.; Guillarme, D.; Fekete, S., Impact of organic modifier and temperature on protein denaturation in hydrophobic interaction chromatography. Journal of Pharmaceutical and Biomedical Analysis 2016,131, 124-132.
7. Goyon, A.; D’Atri, V.; Colas, O.; Fekete, S.; Beck, A.; Guillarme, D., Characterization of 30 therapeutic antibodies and related products by size exclusion chromatography: Feasibility assessment for future mass spectrometry hyphenation. Journal of Chromatography B 2017,1065-1066, 35-43.
8. Wakankar, A.; Chen, Y.; Gokarn, Y.; Jacobson, F. S., Analytical methods for physicochemical characterization of antibody drug conjugates. mAbs 2011,3 (2), 161-172.

Scan to Follow BioNV
Copyright Notice
Bio New Thinking BioNV is an open knowledge sharing platform focused on biopharmaceutical R&D knowledge. All articles from this public account are intended for learning, communication, and knowledge sharing. If there are any infringements, please scan the QR code below to contact us for removal. Reproduced articles represent only the author’s views and do not reflect the position of this public account.

Scan for Communication/Submission/Consultation/Cooperation