
Introduction
Antibody-drug conjugates (ADCs) are “biological bullets” formed by conjugating cytotoxic drugs (payloads) to antibodies via a “linker.” They consist of five components: target, antibody, linker, payload, and conjugation method. Each part is crucial to the function of ADCs, but today we will focus on the linker that connects the antibody and payload, examining the extent of exploration into linkers.
Previous Articles:
ADC Detailed Series – Target Section
ADC Detailed Series – Antibody Section
Characteristics of Linkers
For ADC drugs, the linker must fulfill two tasks: first, it must ensure good stability of the ADC in the bloodstream; second, it must ensure that the ADC can precisely release the payload at the target site.
Therefore, the linker is required to have the following three characteristics:
First, the linker must have good stability to maintain the drug concentration of the ADC in the bloodstream and not release the cytotoxic drug before it reaches the target, thereby minimizing off-target effects and enhancing the safety of the ADC drug;
Second, the linker should allow for rapid release of the effective payload at the target site after internalization;
Third, the linker should have appropriate hydrophilicity/lipophilicity to enhance the binding of the effective payload and reduce immunogenicity[1].
Classification of Linkers
Linkers for ADCs are classified into two types: non-cleavable linkers and cleavable linkers (Table 1). Non-cleavable linkers remain intact during intracellular metabolism. ADCs with this type of linker require the degradation of the antibody in lysosomes to release the effective payload; cleavable linkers can split during intracellular metabolism, producing metabolites that contain the cytotoxic agent, which may have partial linker characteristics[2].
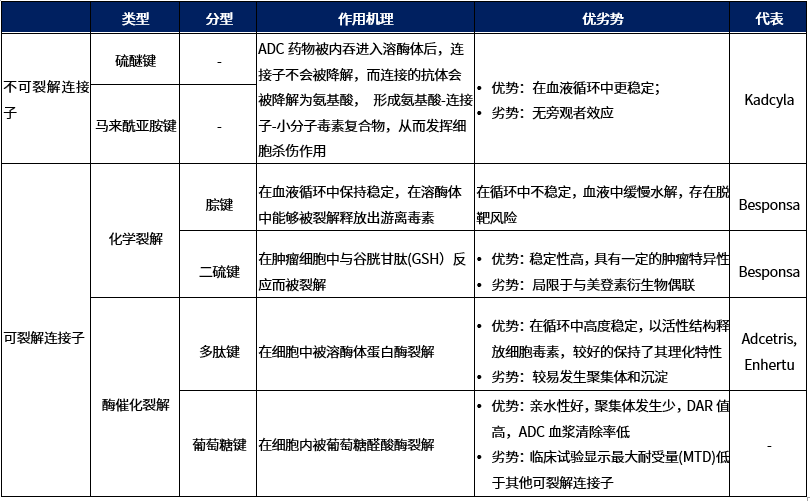
Table 1. Classification of Linkers
Characteristics of Non-Cleavable Linkers
Non-cleavable linkers are divided into two categories: thioether or maleimide-based linkers (MC), which are composed of stable bonds that prevent protein hydrolysis (Figure 1). ADCs with this type of linker rely on lysosomal enzyme degradation to release the internalized effective payload, leading to the simultaneous separation of the linker[3].
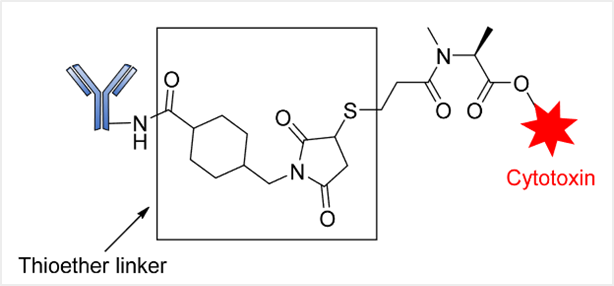
Genentech/Immunogen has successfully explored the application of this linker, such as in the ADC drug trastuzumab emtansine (T-DM1 or Kadcyla®), which targets HER2-positive metastatic breast cancer. This ADC contains a non-cleavable SMCC (N-succinimidyl-4-(maleimidyl) cyclohexane-1-carboxylate) linker that connects the DM1 cytotoxic agent to trastuzumab.
Characteristics of Cleavable Linkers
1. Chemically Cleavable Linkers
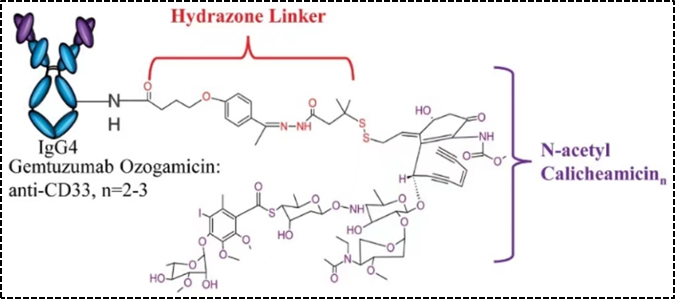
Figure 2. Structural Schematic of Mylotarg
Another type is reducible linkers—disulfide bonds, which are dependent on reduced glutathione. Compared to the level of glutathione in plasma (~5μmol/L), the level of glutathione in the cytoplasm is much higher (1–10 mmol/L), thus the reducible disulfide bond linker is relatively stable in the bloodstream, and glutathione inside the cell reduces the disulfide bond linker to release the effective payload.
2. Enzymatically Cleavable Linkers
Unlike chemically unstable linkers, enzymatically cleavable linkers utilize unique high concentrations of hydrolytic enzymes in cells to cut and release the drug, achieving clinical success in controlling drug release.
The first type is peptide linkers, which mainly include dipeptide linkers and tetrapeptide linkers. The cleavage mechanism of peptide linkers is that after ADC internalization through endocytosis and transport to lysosomes, cathepsin B selectively cleaves the linker, releasing the effective payload.
Among the marketed ADC drugs, the most commonly used dipeptide linkers include Val-Cit and Val-Ala dipeptides, both of which have comparable stability and cellular activity. However, due to precipitation and aggregation, Val-Cit is difficult to achieve a high drug-to-antibody ratio (DAR), whereas Val-Ala linker can achieve a DAR of up to 7.4 with limited aggregation (<10%). Compared to Val-Cit, Val-Ala has higher hydrophilicity, making this linker more advantageous in the context of lipophilic effective payloads, such as PBD dimers. Marketed ADC drugs using the above dipeptide linkers include Adcetris (Figure 3), Polivy, Padcev, and Disitamab Vedotin (RC48).[4].
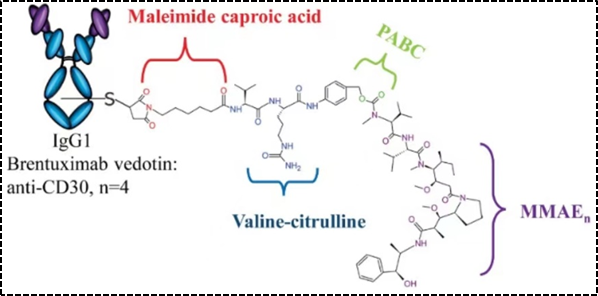
Figure 3. Structural Schematic of Adcetris
In addition to dipeptide linkers, the tetrapeptide Gly-Gly-Phe-Gly has also been successfully applied to ADC drugs. Compared to dipeptides, tetrapeptide linkers are more stable in the bloodstream, and the marketed ADC drug Enhertu (Figure 4) uses this type of linker. The HER-2 targeted ADC drug SHR-A1811 from Hengrui Pharmaceutical, which is about to be launched, also uses the above tetrapeptide linker.
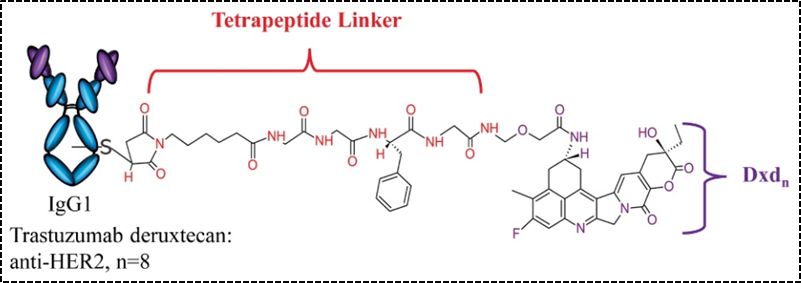
Figure 4. Structural Schematic of Enhertu
The second type of enzymatically cleavable linkers is glycosidase linkers, which mainly include β-glucosidase linkers and β-galactosidase linkers. ADCs sensitive to β-glucuronidase are covalently linked with the cytotoxic drug and antibody by combining β-glucosidase linkers with self-eliminating PABC spacer molecules. Similar to β-glucuronidase, β-galactosidase is overexpressed in certain tumors, and it hydrolyzes β-galactosidic bonds in tumors to release drugs. The difference is that β-galactosidase is only present in lysosomes, while β-glucuronidase is expressed in lysosomes and also in the microenvironment of solid tumors.[5].
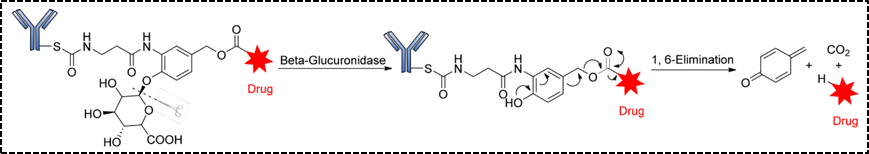
Figure 5. Structure and Release Mechanism of β-Glucuronidase Linker
Conclusion
Linkers affect the stability, toxicity, pharmacokinetics, and pharmacodynamics of ADCs. Therefore, careful selection of the appropriate linker is crucial in ADC design. Meanwhile, the development of new linkers is progressing steadily, which will further enrich the design strategies for ADCs and enhance their clinical value.
References:
[1] Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018 Jan;9(1):33-46.
[2] Sheyi R, de la Torre BG, Albericio F. Linkers: An Assurance for Controlled Delivery of Antibody-Drug Conjugate. Pharmaceutics. 2022 Feb 11;14(2):396.
[3]Kostova V, Désos P, Starck JB, Kotschy A. The Chemistry Behind ADCs. Pharmaceuticals (Basel). 2021 May 7;14(5):442.
[4] Dean AQ, Luo S, Twomey JD, Zhang B. Targeting cancer with antibody-drug conjugates: Promises and challenges. MAbs. 2021 Jan-Dec;13(1):1951427.
[5] Su Z, Xiao D, Xie F, Liu L, Wang Y, Fan S, Zhou X, Li S. Antibody-drug conjugates: Recent advances in linker chemistry. Acta Pharm Sin B. 2021 Dec;11(12):3889-3907.
* This article is intended to provide scientific information to medical professionals and does not represent the views of this platform.


