
Antibody-drug conjugates (ADC) consist of antibodies, linkers, and small molecule cytotoxic drugs, combining high specificity and high cytotoxicity advantages. However, their structural diversity and complexity, along with the low content of released small molecule toxins in the circulatory system, pose significant challenges for pharmacokinetic studies. This article will discuss the mechanisms, design, safety features, and risk management of ADC drugs to help clinicians better understand them.
1. Introduction to Antibody-Drug Conjugates (ADCs)
Antibody-drug conjugates (ADCs) are targeted biopharmaceuticals composed of antibodies, linkers, and cytotoxic drugs. They link target-specific monoclonal antibodies with highly lethal cytotoxic drugs via specific linkers. ADCs typically include three components: highly specific and high-affinity antibodies; highly stable linkers; and effective small molecule cytotoxic drugs.

Composition of ADC Technology
ADC drugs primarily target tumors and are a class of innovative anti-tumor drugs with unique mechanisms of action. Currently, ADC drugs have demonstrated excellent clinical efficacy in solid tumors such as breast cancer, gastric cancer, urothelial carcinoma, and lung cancer, as well as in hematological malignancies.
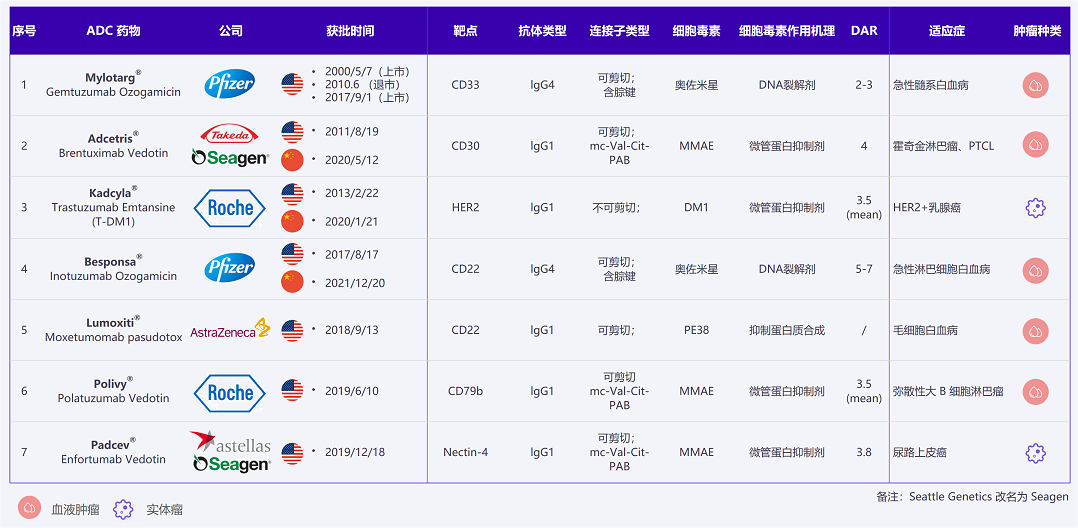
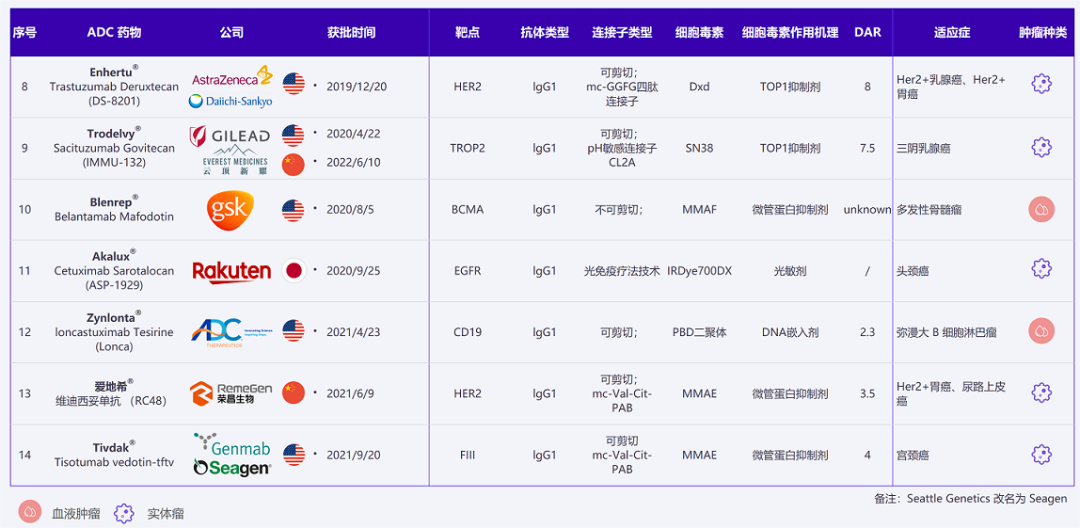
Currently Approved ADC Drugs and Indications Worldwide (Image Source: Dingxiangyuan Insight)
2. Mechanisms and Design of Antitumor ADC Drugs
Once inside the body, ADC drugs specifically bind to target cells expressing corresponding tumor antigens. ADCs enter tumor cells through endocytosis and are then degraded in lysosomal proteins or under low pH conditions, releasing small molecule cytotoxic drugs in an active form to effectively kill tumor cells by damaging microtubules or DNA.

Mechanism of Action of ADC Drugs
Based on the above mechanisms, the design key points of ADC drugs mainly include three aspects:
1. Selection of Target/Antigen and Antibody:
The selection of targets is the starting point for designing ADC drugs, determining which tumor indications the ADC will target, and potentially influencing the choice of cytotoxic drugs to be conjugated.
The basic premise for target selection is high expression in tumor cells and low or absent expression in normal cells, while the choice of antibody types will more often consider selecting IgG1, which can induce various immune responses after binding to target cells compared to other IgGs. During the design process of the antibody, multiple indicators such as affinity and internalization efficiency need to be comprehensively considered without significant shortcomings.
2. Selection of Cytotoxic Drugs:
Currently, only a few cytotoxic drugs and their derivatives are used in the design of ADCs, primarily divided into two categories: microtubule inhibitors/stability disruptors and DNA groove-targeting drugs like calicheamicin and its derivatives. Besides high toxicity, other properties such as hydrophilicity/hydrophobicity balance and good stability are also crucial.
Linkers must ensure that ADC drugs do not break apart while circulating in the bloodstream, while also ensuring that cytotoxic drugs can be released once inside tumor cells, reducing side effects while ensuring efficacy.
3. Risk Mechanisms of ADC Drugs
From the structure, mechanisms, and design of ADC drugs, it can be seen that the toxicity risks of ADC drugs mainly lie in the following aspects:
1. Target Toxicity of Antibody Molecules:
The ideal target for ADCs is one that is highly expressed in tumor cells but low or absent in normal cells. However, in reality, almost all protein targets are expressed in normal cells, bringing risks to the development of ADC drugs.
Off-target toxicity is primarily related to the stability of the linker. Commonly used linkers in ADC drugs include chemical cleavage-type hydrazone bonds, disulfide bonds, enzyme-cleavable dipeptides, and β-glucuronic acid. The stability of the linker directly affects the unintended dissociation of cytotoxic drugs; unstable linkers, once broken, can lead to the release of small molecule cytotoxic drugs, resulting in a certain degree of off-target toxicity.
3. Toxicity of ADC Molecules:
Once the antibody and small molecule cytotoxic drugs are integrated through the linker, the Drug-Antibody Ratio (DAR) will affect the toxicity of ADC drugs. There may also be unbound cytotoxic drugs in the DAR mixture, causing off-target toxicity. Additionally, the addition of linkers and cytotoxic drugs to antibody molecules may lead to changes in the spatial structure of the ADC, differing from that of naked antibodies, resulting in unintended toxicity.
Common Toxicities and Risk Management of ADC Drugs
The adverse reactions of ADC drugs are classified according to the affected organ systems: hematological adverse reactions, infusion-related reactions, gastrointestinal reactions, peripheral neurotoxicity, hepatic toxicity, pulmonary toxicity, cardiac toxicity, skin reactions, infections, metabolic toxicity, ocular toxicity, etc. The details are summarized in the table below:

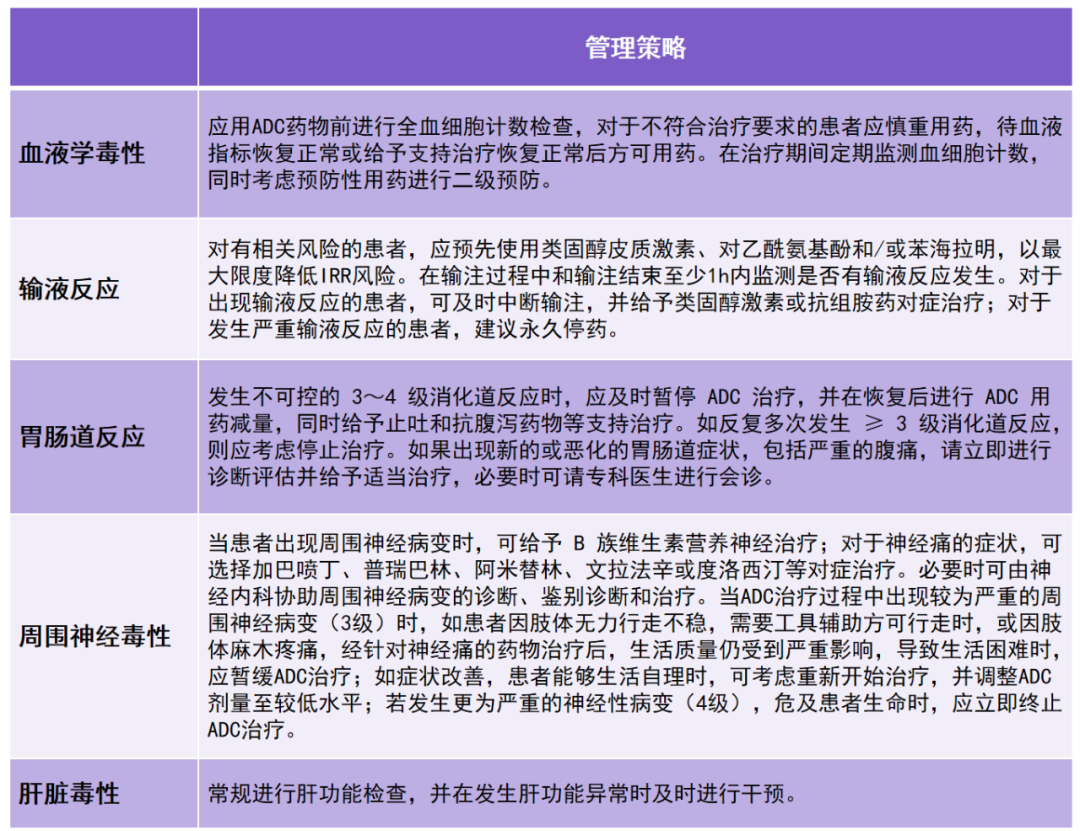
Common Risk Management Strategies for Antitumor ADC Drugs are summarized in the table below:
Conclusion
In recent years, ADC drugs have experienced unprecedented development. It is essential for clinicians to deeply understand the molecular characteristics and mechanisms of ADC drugs and to rationally use them in clinical applications while effectively managing adverse reactions to maximize the clinical efficacy of these drugs, which significantly impacts patient survival outcomes.
Antibody-drug conjugates (ADCs) consist of antibodies, linkers, and small molecule cytotoxic drugs, and their research and application in the field of tumor treatment are flourishing.
Compared to previous tumor treatment drugs, ADCs combine the powerful lethality of small molecule drugs with the high specificity of monoclonal antibodies, accurately delivering drugs to tumor cells while avoiding damage to normal cells, thus reducing adverse reactions during treatment.
However, with the increasing interest in ADC research, more issues have emerged, and some drug-related adverse reactions have been observed during clinical use, including serious events.
The associated risks of ADCs mainly involve off-target toxicity of cytotoxic small molecule drugs, and the stability of the linker-antibody connection is a challenge in ADC drug design. Early release of cytotoxic drugs during blood circulation leads to related toxicities.
As the variety of ADC drugs increases and their application scope expands, associated immunogenicity issues are gradually surfacing, affecting the safety and efficacy of the drugs.
The author of this article starts with the mechanisms of ADC drugs, analyzing the main sources and risk characteristics of adverse reactions. Various clinical manifestations of toxic reactions observed during the research process are listed, and the causes are speculated.
The author recommends treatment plans and management strategies for toxic reactions, and grades the management of some toxic response strategies. The article is concise and targeted, providing strong guidance for clinical applications, and can serve as a reference for oncologists.
✩ This article is for reference by healthcare professionals only
Planned by: Wang Binru
Submission and Cooperation: [email protected]
Image Source: Zhanzku Hailuo PLUS
References:
1. Chinese Anti-Cancer Association, Clinical Research Committee for Antitumor Drugs, National Expert Committee for Clinical Application Monitoring of Antitumor Drugs, National Quality Control Center for Tumors, Breast Cancer Expert Committee, etc. Expert Consensus on the Clinical Application of Antibody-Drug Conjugates for Malignant Tumors (2020 Edition) [J]. Chinese Journal of Medical Frontiers (Electronic Edition), 2021, 13(1): 1-15.
2. Huang Ying, Huo Yan, Wang Xin, et al. Preclinical Safety Evaluation Strategies and Cases for Novel Antibody Drugs. Drug Evaluation Research. (2018)01-0035-06









