Introduction
Antibody-drug conjugates (ADCs) are a new type of highly effective biopharmaceuticals that link antibodies with biologically active small molecule cytotoxic payloads through linkers.
ADCs are designed to improve the therapeutic index (TI) of chemotherapeutic agents by selectively delivering cytotoxic drugs to tumor cells while reducing exposure in normal cells. Despite using a large number of antibodies targeting antigens expressed only on cancer cells (i.e., target cells), dose-limiting toxicities (DLTs) in normal cells/tissues are frequently reported even at suboptimal therapeutic doses. The toxicity mechanisms of ADCs in normal cells/tissues remain unclear, but most DLTs are believed to be target-independent.
In addition to the instability of ADCs leading to premature release of cytotoxic drugs (payloads) in circulation, the uptake/transport of intact ADCs through dependent receptors (FcγRs, FcRn, and C-type lectin receptors) and non-specific endocytic mechanisms may lead to off-target toxicity in normal cells.
This article summarizes the non-targeted uptake of ADCs in normal cells and the potential mechanisms of toxicity, discussing which components of ADCs influence these mechanisms. This information will help deepen the understanding of the potential mechanisms of ADC off-target toxicity and aid in improving the overall therapeutic index (TI) of next-generation ADCs.
Overview
So far, a total of 16 ADC drugs have been approved globally, with one (Trastuzumab duocarmazine) in the application stage for market approval. In recent years, the ADC field continues to expand with a significant increase in IND applications submitted to the FDA. Currently, over 900 different ADCs are in various stages of research and development, primarily targeting hematologic malignancies and solid tumors.
In the development of ADC drugs, despite the use of antibodies targeting tumor-specific and/or overexpressed antigens, dose-limiting toxicities (DLTs) at suboptimal therapeutic doses remain a major challenge for the clinical application of ADCs. DLTs lead to relatively narrow therapeutic indices (TIs) and are the main reason limiting dose escalation of ADCs to achieve maximum efficacy.
Table 1 summarizes the preclinical toxicities and clinical DLTs of four ADCs approved by the FDA. According to the data described in Table 1 and other published literature, the reported ADC toxicities in normal cells/tissues are primarily driven by the payloads. Table 2 summarizes the different types of payloads used in ADCs and the major toxicities reported in clinical studies.
Table 1: Summary of preclinical toxicities and clinical DLTs or SAEs of four FDA-approved ADCs
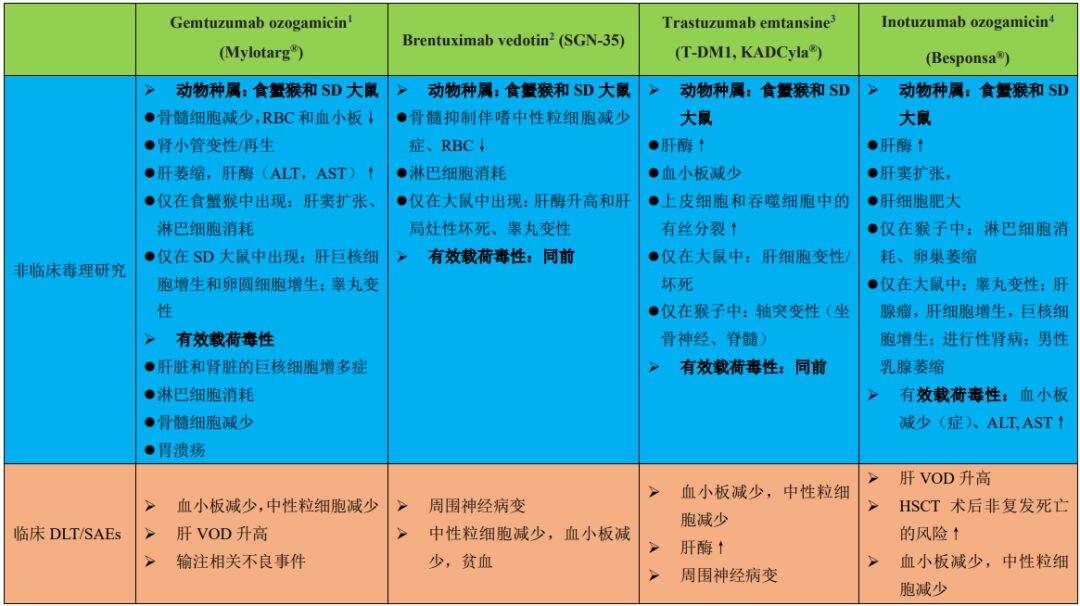
1: Anti-CD33 antibody conjugated through a cleavable hydrazine linker (approved in 2000, Pfizer/Wyeth-Ayerst Laboratories)
2: Anti-CD30 antibody conjugated to MMAE with a protease-cleavable valine-citrulline linker (approved in 2011, Seattle Genetics)
3: Anti-HER2 antibody conjugated to DM1 with an uncleavable SMCC linker (approved in 2013, Genentech)
4: Anti-CD22 antibody conjugated to calicheamicin with an acid-labile butyric acid linker (approved in 2017, Pfizer)
Table 2: Clinical toxicities or adverse events related to ADC payloads

Although ADC toxicity is primarily believed to stem from payloads, the mechanisms of ADC uptake in non-targeted normal cells and the delivery of cytotoxic payloads remain unclear. Only a small amount of ADC accumulation occurs at human targets (tumors) (~0.1% of the administered dose per gram of tumor). Most ADCs remain in circulation or are distributed in normal tissues, undergoing absorption and metabolic degradation, leading to toxicity in normal cells.
The expression of target antigens in normal tissues (albeit at lower levels) may lead to target-dependent uptake of ADCs and subsequent toxicity. For example, the dose-limiting gastrointestinal toxicity (hemorrhagic gastritis) of BMS-182248-01 is associated with the expression of the Lewis-Y target antigen on normal gastric mucosal cells.
It is also important to note that the expression of target antigens in normal cells does not typically predict ADC toxicity. For instance, the clinical toxicity of Trastuzumab emtansine (T-DM1, KADCyla®), an ADC targeting HER2, does not show evidence of toxicity related to these organs despite high HER2 expression levels in important organs such as the heart and kidneys. Severe thrombocytopenia is a common DLT of T-DM1, which is largely considered a target-independent effect due to the absence of HER2 expression on platelets or megakaryocytes in circulation.
In addition to target antigen expression, factors such as the internalization rate of target antigens, circulation/transport kinetics, intrinsic sensitivity to payloads, and the in vivo distribution of ADCs to normal cells/tissues may determine ADC toxicity. Generally, tissues with high perfusion and vascular leakage (incomplete and/or absent basement membranes) such as the liver, bone marrow, and spleen are expected to have higher IgG/ADC distribution and exposure compared to other normal tissues.
Some ADCs have the same payload and linker but target different antigens, exhibiting similar maximum tolerated doses (MTDs) and similar toxicities in normal cells/tissues. The most common ADC toxicities are independent of target antigen expression. For example, neutropenia is usually considered a DLT for most MMAE-based ADCs (with cleavable linkers). Similarly, ocular (corneal) toxicity is a DLT for several ADCs containing DM4.
Moreover, ocular toxicity has been observed in multiple MMAF-based ADCs targeting different antigens. This leads to the conclusion that ADC toxicity is largely off-target effects, further indicating the potential effects of drug-linker combinations (ADC platforms) on specific off-target toxicities. Therefore, there is ample reason to believe that all three components of ADCs (i.e., monoclonal antibodies, payloads, and linkers) can contribute to toxicity in normal cells/tissues.
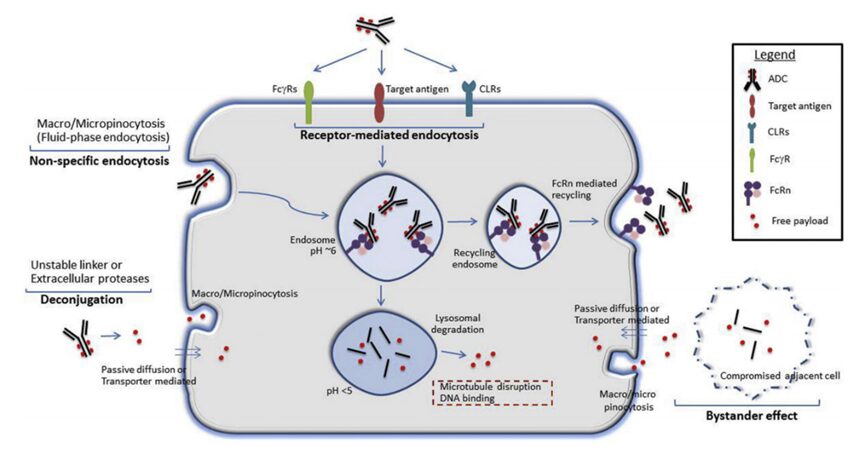
The potential mechanisms of ADC or free payload uptake in normal cells are illustrated in Figure 1. Different receptor-dependent and non-receptor-dependent (non-specific endocytosis) mechanisms may facilitate the uptake of intact ADCs and/or the release of free payloads in normal cells. In addition, the premature release of payloads due to linker-payload instability in circulation may also lead to toxicity unrelated to targets. Understanding the mechanisms of target-independent ADC uptake and toxicity in healthy normal cells is crucial for improving ADC technology.
Figure 1: Potential mechanisms of ADC or free payload uptake in normal cells.
Target antigens may be expressed on normal cells, facilitating target-dependent uptake of ADCs. Additionally, other receptors that bind to the conserved Fc region of IgG antibodies, such as FcγRs, neonatal Fc receptor (FcRn), and C-type lectin receptors (CLRs), may also facilitate the internalization of ADCs in normal cells. Non-specific endocytic mechanisms, such as macropinocytosis or micropinocytosis, may also contribute to the internalization of intact ADCs or free payloads (released extracellularly due to linker-payload instability or extracellular protease activity).
Free payloads can also enter normal cells through other mechanisms, such as passive diffusion (if membrane-permeable), non-specific endocytosis, or specific transporters (if substrates of membrane transporters). Furthermore, antigen-positive target cells can also mediate toxicity by releasing payloads into the local environment, which are subsequently absorbed by antigen-negative normal cells through passive diffusion, paracrine uptake, or other non-specific endocytic mechanisms (bystander effect).
Non-Targeted Dependent ADC
Uptake and Potential Toxicity Mechanisms
1Linker-Payload Linkage Instability
Linker-payload instability can lead to premature release of payloads into the bloodstream, resulting in off-target toxicity of ADCs. The choice of linker is one of the main driving factors behind the stability of ADCs. First-generation ADCs have acid-labile bonds (e.g., hydrazine) that are stable at neutral pH in plasma but release payloads in the lysosomes at lower pH after ADC internalization. These early ADCs often suffered from poor plasma stability, with free payloads detectable in circulation (Figure 1).
The introduction of uncleavable linkers has alleviated linker cleavage issues in some cases, improving preclinical safety. The reduced toxicity of uncleavable linker types is believed to result from decreased release of free cytotoxic payloads.
However, not all targets are suitable for uncleavable ADCs, as complete antibody degradation is required to release linker-payloads. ADCs with cleavable linkers may also enhance efficacy through bystander effects, making them preferable for low-copy number, heterogeneous tumors with low expression or internalization rates of antigens. It is also important to note that, in addition to the cleavability of the linker, the membrane permeability of the released payloads may also influence the potential off-target cytotoxicity in normal cells, thereby impacting TI.
Conjugation sites may affect the stability and pharmacokinetic characteristics of ADCs. Traditional non-specific conjugation methods use surface-exposed amino acids, such as lysine or cysteine, resulting in highly heterogeneous ADCs (drug-to-antibody ratio [DAR], 0 to 8), increased aggregation, and reduced plasma stability. Therefore, non-specifically conjugated ADCs may also contribute to increased target-independent uptake and toxicity in normal cells.
Neutropenia is a significant target-independent DLT of ADCs, linked to the instability of cleavable linkers in plasma, associated with systemic release of membrane-permeable free payloads. Neutropenia is a common toxicity of many adenylate kinases through protease-cleavable valine-citrulline linkers, such as Brentuximab vedotin (ADCetris, Seattle Genetics), ASG-5ME (Agensys), Glembatumumab vedotin (Celldex Therapeutics), Indusatumab vedotin (Millennium Pharmaceuticals), Polatuzumab vedotin (Genentech), and PSMA ADC (Progenics Pharmaceuticals).
Depending on the chemical nature of the linker, the valine-citrulline linker can undergo intracellular cleavage mediated by cysteine proteases in lysosomes. Local serine proteases secreted by differentiated neutrophils in the bone marrow contribute to the cleavage of extracellular VC linkers and release membrane-permeable MMAE, leading to cytotoxicity in neutrophil differentiation in the bone marrow.
Similarly, peripheral neuropathy (PN) is another significant target-independent toxicity associated with microtubule-inhibiting ADCs (regardless of target antigen). PN is believed to be driven by linker-payload instability, associated with premature release of membrane-permeable free payloads (microtubule inhibitors) in circulation. Microtubule inhibitors disrupt interphase microtubule function, which is critical for the active transport of essential proteins from neuronal cell bodies to distal synapses, ultimately leading to peripheral neuropathy. PN is a common adverse event for nearly all membrane-permeable ADCs (DM-1 and DM-4) linked to cleavable linkers.
It is noteworthy that PN observed clinically is not always predicted in preclinical animal models. For example, preclinical toxicology studies of VC-MMAE-based ADCs did not monitor PN. For other non-MMAE ADCs containing microtubule inhibitors, such as DM1 or DM4, PN has been observed in preclinical species with good clinical predictability.
Bystander Effect: In addition to the direct cytotoxicity of ADCs taken up by antigen-positive cells, free payloads from ADCs may also exert cytotoxicity on adjacent antigen-negative cells through a phenomenon known as the bystander effect. In antigen-expressing target cells, the uptake and degradation of ADCs in lysosomes release free payloads into the cytoplasm. Subsequently, free payloads can passively enter the extracellular space (membrane-permeable, lipophilic payloads) or be released due to loss of membrane integrity (after target cell death). Released free payloads may enter antigen-negative cells through passive diffusion, transporter-mediated uptake, or other non-specific endocytic mechanisms, causing cytotoxicity (Figure 1).
The bystander effect of ADCs is often associated with increased tumor killing, particularly for tumors with heterogeneous antigen expression. Through in vitro colony formation assays and co-culture systems, as well as in vivo xenograft models, its effect on the efficacy and effectiveness of ADCs with membrane-permeable payloads has been demonstrated. However, the increased cell permeability required to achieve the bystander effect may also lead to off-target toxicity.
Compared to uncleavable, poorly permeable payloads, released payloads can penetrate normal tissues, leading to increased toxicity. Recent advances in ADC technology enable cytotoxic payloads to be metabolized into membrane-impermeable metabolites (e.g., Dolaflexin) within tumor cells. This approach can control the bystander effect while retaining beneficial chemical properties to kill tumor cells, significantly reducing systemic toxicity to normal cells.
2Non-Specific Endocytosis
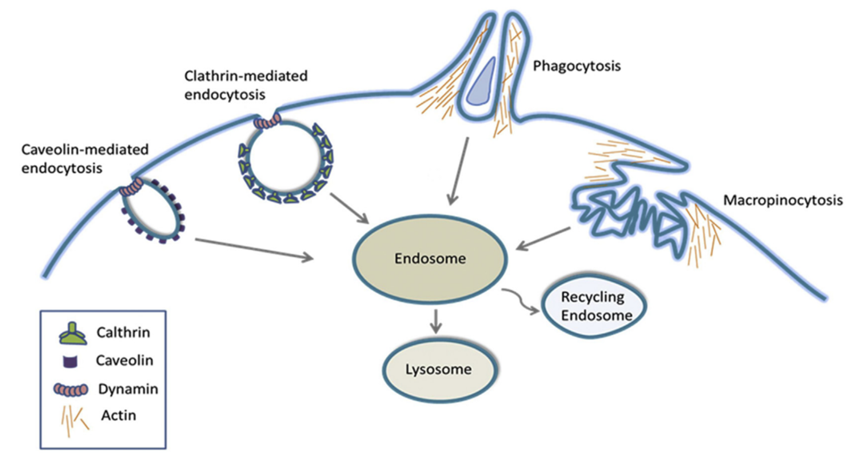
Endocytosis is an important process for cell uptake of nutrients, regulating transmembrane dynamics, and synaptic vesicle recycling. Endocytosis can also play a significant role in the uptake and distribution of large molecules, including IgG/ADCs, in normal cells. Endocytosis is broadly classified into phagocytosis (particle internalization) and pinocytosis (soluble molecule internalization, also known as liquid-phase endocytosis). Additionally, based on the size of the endocytic vesicles formed, endocytosis is divided into macro and micro endocytic processes. Table 3 lists key features of major endocytic mechanisms that may lead to non-specific uptake of IgG/ADCs.
Table 3: Key Features of Major Endocytic Mechanisms
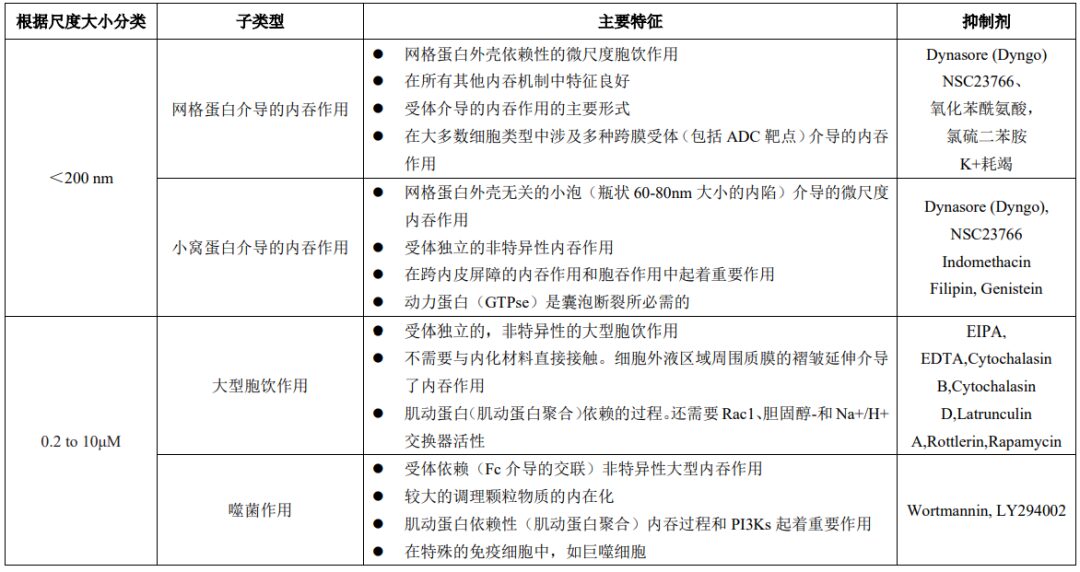
“Macroscopic” endocytosis includes phagocytosis and macropinocytosis, which involve the internalization of large particles and large volumes of fluid, respectively. Phagocytosis involves the uptake of larger particles, causing deformation of the cell membrane (local rearrangement of actin). Immune complexes containing ADCs or ADC aggregates may also be absorbed through this process.
Similar to phagocytosis, macropinocytosis is also an actin-dependent process that involves the extension of membrane folds around relatively large areas of extracellular fluid (rather than particles) to mediate endocytosis. Microscopic endocytic processes involve the uptake of small vesicles smaller than 200 nm.
These processes often require specialized coat proteins, such as clathrin or caveolin (Figure 2). The binding of ligands to specific membrane receptors initiates a series of signaling events leading to the recruitment of specific adapter proteins to achieve the formation of clathrin-coated vesicles. These newly formed vesicles are cleaved by dynamin (GTPase enzymes) and released for further intracellular transport.
Caveolin-mediated endocytosis involves flask-shaped structures (caveolae) formed by membrane-coating protein caveolin, which also relies on dynamin to cleave the vesicles. Caveolin-mediated uptake plays a major transport role in many cell types, particularly dominating in endothelial cells.
Figure 2: Key Structural Features of Macroscopic and Microscopic Endocytic Processes
Overall, the aforementioned macroscopic and microscopic endocytic processes may facilitate the entry of ADCs into normal cells. Regarding the toxicity of ADCs to normal cells, non-specific endocytic mechanisms such as caveolin-dependent endocytosis, macropinocytosis, and phagocytosis are potential important mechanisms. It is also clear that endocytic mechanisms and overall endocytic rates differ among normal tissues and cell types.
Many specialized immune cells (including macrophages and dendritic cells) have higher endocytic rates. For example, Kupffer cells (resident macrophages in the liver) play a major role in the non-specific uptake and clearance of immune conjugates, including ADCs. Since endothelial cells are located at the interface between blood vessels and interstitial compartments, they also have a higher rate of macromolecular endocytosis. Understanding the endocytic rates of different normal cells/tissues is valuable for understanding the role of non-specific endocytosis as a potential mechanism for ADC uptake and toxicity.
Factors influencing non-specific endocytosis of IgG/ADCs: The physicochemical properties of large molecules may influence endocytosis in normal cells/tissues. The molecular charge of the IgG/ADC surface is one of the key parameters influencing antibody tissue distribution and PK among many parameters. Positively charged molecules are attracted to negatively charged groups on mammalian cell membranes and extracellular matrices (heparan sulfate proteoglycans). This increases the local concentration of ADCs, leading to more non-specific endocytic uptake in normal tissues/cells.
Overall, the increase in net positive charge of IgG antibodies leads to increased tissue distribution and increased plasma clearance rates, while the decrease in net positive charge leads to decreased tissue distribution. Importantly, a change in at least one or more units of isoelectric point (pI) is sufficient to produce measurable changes in tissue distribution and PK. These conclusions may also apply to ADCs, supporting the hypothesis that the charge of ADCs may influence non-specific endocytosis in normal cells. Therefore, charge modification, through reducing positive charges or balancing the overall surface charge distribution, is a method to consider when designing ADCs.
However, it is worth noting that, similar to normal tissues, charge modifications may also affect the target antigen-dependent ADC uptake required for tumor cell efficacy. Optimizing the surface charge of ADCs to reduce normal cell uptake while retaining target-mediated uptake in tumor cells could be beneficial for improving TI.
The hydrophobicity of ADCs may also play a role in their non-specific uptake by normal cells. Many drug-linker combinations used for ADCs are hydrophobic, imparting significant hydrophobicity to antibodies, especially for ADCs with high DAR. The increased hydrophobicity of high DAR ADCs can promote aggregation and accelerate non-specific clearance. Similar to hepatocytes, high DAR ADCs may be rapidly cleared by other normal cells with high non-specific endocytic capacity, leading to off-target toxicity.
Non-specific endocytosis (especially macropinocytosis) is considered a pathway for normal corneal epithelial cells and megakaryocytes to uptake ADCs, leading to ocular toxicity and thrombocytopenia, respectively. Similarly, macropinocytosis-mediated internalization can reduce the toxicity of ADCs (AGS-16C3F) to megakaryocytes (thrombocytopenia).
3Receptor-Mediated Uptake Mechanisms
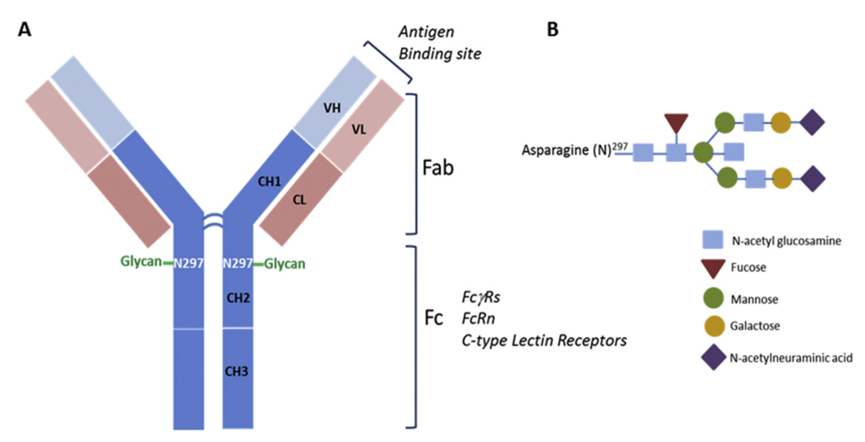
The target-dependent uptake and toxicity of ADCs can also be mediated by different receptors that recognize the Fc (fragment crystallizable) region of IgG in ADCs (Figure 3). The constant domain of IgG is structurally highly conserved, allowing interaction with other components of the immune system through Fc receptors, thereby initiating effector immune functions. Although Fc-mediated effector functions are generally not required to achieve the efficacy of ADCs, the recognition and binding of Fc receptors to the ADC antibody (IgG) components can mediate non-target internalization in normal cells.
Figure 3: Schematic Diagram of the Structure of the Fc Region of ADC IgG
Fcγ Receptors (FcγRs): FcγRs play a crucial role in mediating antibody-mediated effector functions (such as antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), phagocytic cytokines (IFN-γ and TNF-α), and other IgG immune complexes and released cytokines) in connecting cellular and humoral immune responses. These effector functions play a significant role in regulating the efficacy of several therapeutic IgG antibodies. FcγR-mediated effector functions are essential for target-related efficacy but may contribute to off-target uptake and toxicity in normal cells.
Therefore, understanding the biology of FcγRs, the expression patterns in normal cells/tissues, and the physicochemical factors that facilitate FcγR binding to ADCs is crucial for understanding potential off-target toxicity mechanisms. FcγRs are mainly divided into activating (FcγRI, FcγRIIa, FcγRIIc, FcγRIIIa, and FcγIIIb) and inhibitory (FcγRIIb1/b2) receptors, based on the types of signaling pathways initiated after receptor crosslinking.
Activating FcγRs positively regulate effector functions through interactions with immunoreceptor tyrosine activation motifs (ITAMs), while inhibitory receptors negatively regulate IgG-mediated effector functions, including endocytosis/phagocytosis through interactions with immunoreceptor tyrosine inhibitory motifs (ITIMs). Besides immune cells, the expression of different FcγRs is also observed in several other normal cell types, including epidermal keratinocytes, sensory neurons, mesangial cells, osteoclasts, endothelial cells, fibroblasts, salivary gland epithelial cells, various cell types in the kidneys and eyes, megakaryocytes, platelets, and differentiated bone marrow-derived immature cells, including hematopoietic stem/progenitor cells.
FcγR-Mediated IgG/ADC Internalization: FcγRs are not only important molecules mediating the effector functions of IgG antibodies but also one of the most characteristic endocytic receptors on the cell surface, playing a role in the internalization/clearance of IgG-opsonized antigens in circulation. The binding of FcγRs to the Fc region induces the aggregation/crosslinking of IgGs on the cell surface and initiates downstream signaling events leading to the phosphorylation and activation of kinases such as PI3K, p70S6K, and Akt. These are directly involved in the reorganization of the actin cytoskeleton and membrane remodeling for the formation of pseudopodia and phagosomes. Similar mechanisms may also apply to FcγR-mediated ADC internalization, contributing to non-target-dependent toxicity in normal cells.
The toxicity of ADCs in normal cells is associated with FcγR-mediated ADC uptake. Although the expression patterns of FcγRs in normal healthy cells/tissues correspond to several reported ADC off-target toxicities, the role of FcγRs in mediating ADC off-target toxicity is primarily considered a potential mechanism for hematological toxicity (blood cell toxicity). Hematological toxicity is the most common off-target toxicity of ADCs containing MMAE, calicheamicin, and DM-1. In clinical studies, T-DM1-induced thrombocytopenia is a DLT.
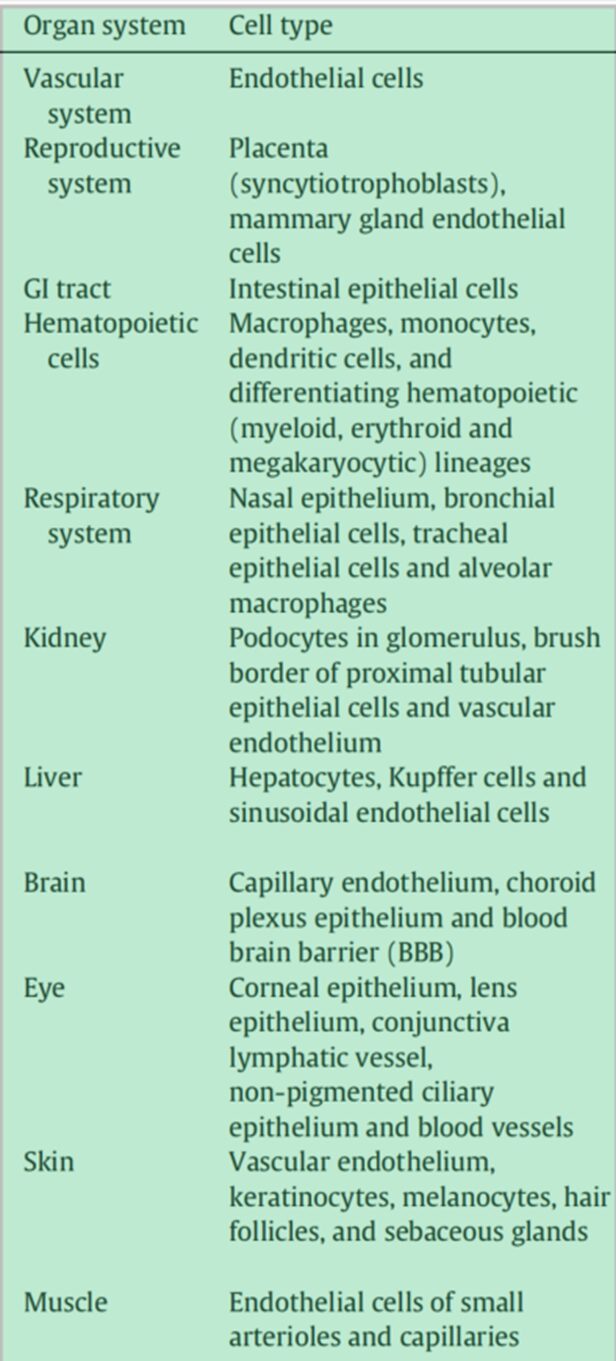
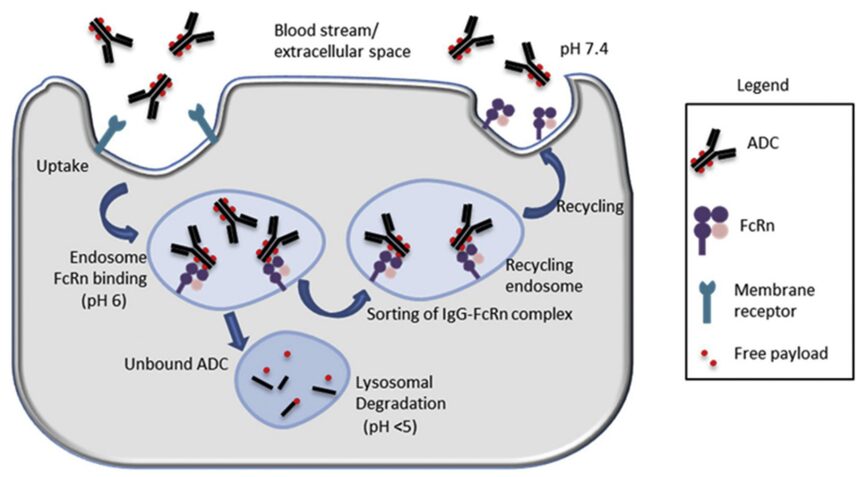
Neonatal Fc Receptor (FcRn): FcRn is a member of the MHCI class glycoproteins that specifically binds to the Fc domain and plays a critical role, characterized by a long half-life (~21 days). Unlike other Fc receptors, FcRn interacts with ligands in a pH-dependent manner, exhibiting high-affinity binding at mildly acidic pH (~6.5). This pH dependence is believed to be the key mechanism by which FcRn prolongs the half-life of IgG/ADCs. FcRn is widely expressed in many normal adult tissues/cell types (Table 4).
In particular, vascular endothelial cells and myeloid-derived hematopoietic cells (antigen-presenting cells) play a dominant role in FcRn-mediated immune responses, thereby influencing the metabolism and PK of IgG/ADCs. FcRn expressed in polarized epithelial cells (such as intestinal epithelial cells and proximal tubular epithelial cells) also facilitates the bidirectional endocytosis of IgG or immune complexes.
The expression patterns of FcRn may vary among different species, leading to differences in IgG binding affinity between humans and other preclinical species. While human FcRn only binds to human IgG, mouse FcRn is highly promiscuous, binding IgGs from multiple species (including humans) with a higher affinity than mouse IgG (approximately 10 times). The binding affinity of human IgG to cynomolgus monkey FcRn is also twice that of human FcRn.
Table 4: Expression of FcRn in Different Normal Cells/Tissues
FcRn Binding and Its Potential Role in ADC Toxicity: ADCs primarily undergo non-specific fluid-phase endocytosis, binding to FcRn in acidified early endosomes, and then, in the neutral pH extracellular space or circulation, the FcRn-ADC complexes are transferred away from lysosomal degradation and recycled back to the cell surface for ADC release. During each endocytic cycle, only unbound ADCs are transported to lysosomes for degradation and payload release (Figure 4).
From a safety perspective, FcRn binding is also important for reducing the accumulation and degradation of ADCs in normal cells to release cytotoxic payloads. Therefore, modifications for FcRn binding in normal cells with significant FcRn expression may be a useful approach to overcome adverse toxicities/adverse events.
Figure 4: The Role of FcRn in the Circulation of ADCs
C-Type Lectin Receptors (CLRs): CLRs are a large, highly conserved, and well-characterized family of endocytic receptors. Type I CLRs are calcium-dependent and have multiple (6 to 8) carbohydrate recognition domains (CRDs), also containing cysteine-rich and fibronectin-like domains. Members include macrophage mannose receptor (MR, MRC1, CD206), Endo180 (CD280, MRC2, uPAR-associated protein, uPARAP), DEC-205, PLA2R, and DCL-1. Type II CLRs contain a single CRD, which can be calcium-dependent Dectin 2, Mincle, CLECSF8, DCIR, DCAR, BDCA-2, DC-SIGN, MGL, and calcium-independent Dectin 1, CLEC5A, DNGR-1 (CLEC9A).
CLRs can be membrane-bound (mainly) or soluble/secreted and are primarily found on myeloid cells. Increasing evidence also indicates that CLRs have functional expression in various normal epithelial cells and endothelial cells, including dermal microvascular endothelial cells (DMECs), liver sinusoidal endothelial cells (LSECs), perivascular small glial cells, mesangial cells in the kidneys, and corneal epithelial cells.
Notably, although CLRs are expressed in various normal tissues, certain pathophysiological events, such as inflammation and infection (fungal, microbial), have been shown to significantly regulate the expression of these receptors. The important endocytic functions and structural expressions of CLRs in some normal tissues, including common ADC target organs (liver, skin, and cornea), suggest that these receptors may mediate non-target ADC internalization and toxicity in these tissues.
ADC toxicity is associated with CLR binding. Although there is no direct evidence that CLRs play a role in the off-target toxicity of ADCs, mannose receptor (MR)-mediated uptake is considered a potential mechanism of ADC hepatotoxicity. LSECs are highly specialized endothelial cells. Compared to other liver cells, LSECs exhibit significantly higher lysosomal enzyme activity levels, which may further lead to the high degradation of their internalized ligands (including ADCs) and release of degraded substances/cytotoxic payloads into the surrounding compartments.
LSECs rely on MR-mediated uptake of lysosomal enzymes (glycoproteins) to maintain their high degradation capacity. After the release of ligands in early endosomes, rapid internalization of MR-ligand complexes and quick recycling of MRs back to the cell surface may further contribute to the high continuous endocytic capacity of MR-expressing cells. MR-mediated uptake is also an important mechanism for clearing endogenous and therapeutic glycoproteins and immunoglobulins in LSECs.
Additionally, due to the presence of large pores (~50-150nm) surrounded by microtubules (actin) without membranes and basement membranes, LSECs are the most permeable type of endothelial cells in vivo. Therefore, the diffusion of large molecules (including ADCs) into LSECs through pore receptors may also lead to hepatotoxicity.
It is also noteworthy that Kupffer cells express MR and play an important role in the non-specific uptake and processing of ADCs. Therefore, MR-mediated uptake of ADCs in Kupffer cells and the release of cytotoxic payloads into surrounding cells cannot be ruled out, leading to hepatotoxicity.
Conclusion
ADC toxicity primarily affects the bone marrow/hematologic system, liver, eyes, peripheral nerves, kidneys, and serous effusions (vascular leakage syndrome). Receptor-dependent and independent mechanisms may contribute to the understanding of ADC off-target toxicity. These mechanisms may vary depending on different cell/tissue types, depending on the expression and function of key candidate receptors. Common DLTs include thrombocytopenia (mediated by FcγRIIa or macropinocytosis), ocular toxicity (mediated by macropinocytosis), neutropenia (mediated by extracellular proteases), liver injury (mediated by mannose receptors), and peripheral neuropathy (circulation) associated with ADC/payload uptake mechanisms.
In summary, selectively targeting tumor cells expressing ADCs is far more complex than initially anticipated. Simply selecting antibody targets that are low or not expressed in normal tissues and highly expressed in tumors is insufficient to effectively deliver ADC payloads to tumor cells in vivo while minimizing toxicity to normal cells. The pathways of non-specific ADC entry into normal cells may vary by cell type and depend on the characteristics of the ADC itself. To date, not all parameters influencing non-specific ADC absorption have been identified, and the more we learn, the complexity seems to continue to expand.
1Mahalingaiah PK, Ciurlionis R, Durbin KR, Yeager RL, Philip BK, Bawa B, Mantena SR, Enright BP, Liguori MJ, Van Vleet TR. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol Ther. 2019 Aug;200:110-125.
2Pretto F, FitzGerald RE. In vivo safety testing of Antibody Drug Conjugates. Regul Toxicol Pharmacol. 2021 Jun;122:104890. doi: 10.1016/j.yrtph.2021.104890. Epub 2021 Feb 13. PMID: 33587934.
3Johns AC, Campbell MT. Toxicities From Antibody-Drug Conjugates. Cancer J. 2022 Nov-Dec 01;28(6):469-478. doi: 10.1097/PPO.0000000000000626. PMID: 36383910.

Scan the WeChat QR code to add the Antibody Circle editor, and qualified individuals can join the Antibody Circle WeChat group!
Please indicate: Name + Research Direction!

All articles reprinted by this public account are intended to convey more information, and the source and author are clearly indicated. If any media or individual does not wish to be reprinted, please contact us ([email protected]), and we will immediately delete it. All articles represent the author’s views and do not represent the position of this site.