Antibody-drug conjugates (ADCs) are formed by linking monoclonal antibodies that target specific antigens with cytotoxic small molecule drugs, combining the targeting specificity of antibody drugs with the powerful lethality of cytotoxins. The aim is to overcome the issues of weak cytotoxicity of monoclonal antibodies and the high toxicity of potent cytotoxins. However, the concept of ADC drugs has been around for over 100 years, and progress has not been smooth. The clinical development success rate of ADC drugs in the oncology field is 10.8%, lower than that of CAR-T, siRNA/RNAi, and monoclonal antibodies. The first-generation ADC drug Mylotarg developed by Pfizer was withdrawn from the market in 2010 due to safety issues, while Roche’s second-generation ADC drug T-DM1 did not outperform traditional targeted combination chemotherapy. It wasn’t until 2019 that DS-8201 emerged, showcasing a disruptive breakthrough against existing therapies. As of now, 15 ADCs have been approved globally, with 11 ADCs approved after 2019, marking the official entry of the ADC field into a rapid development phase. An article published in 2021 in “Nature Reviews Drug Discovery” even predicted that by 2026, the global market size for approved ADC drugs will exceed $16.4 billion, equivalent to nearly 110 billion RMB.
Despite the advantages of high targeting ability of antibodies and high cytotoxicity of small molecule toxins, toxicity remains a major challenge due to the diversity and complexity of their structures. To date, over 260 ADCs have entered clinical trials in the oncology field, with 92 of them terminated. Nearly one-third of ADCs in clinical trials have toxicity that cannot be tolerated as the reason for termination. Recently, several products have been terminated due to serious safety incidents and fatalities, such as XMT-1536, XMT-2056, and LOTIS-9, which were highly anticipated ADCs. With the continuous optimization of ADC drug technology, the therapeutic window has been widened to some extent, and the therapeutic index has increased, but there is still significant room for optimization.
The toxicity mechanism of ADCs in normal cells/tissues is not yet clear, but it is believed that most dose-limiting toxicities (DLT) are unrelated to the target. In addition to the instability of the linker-drug causing cytotoxic drugs (payloads) to be released prematurely into circulation, receptor-dependent (FcγR, FcRn, and C-type lectin receptors) and receptor-independent (non-specific endocytosis) mechanisms of uptake/transport of ADCs may lead to off-target toxicity in normal cells. Some literature suggests that the toxin payload of ADC drugs is consistent with the measured maximum tolerated dose (MTD) and similar compounds, with no significant improvement observed, which may be related to the fact that most ADCs currently use random coupling and cleavable linkers, as well as insufficient drug stability leading to off-target toxicity. According to current data, meta-analysis shows that different toxins have distinct toxicity characteristics and are unrelated to the target antigen (Table 1). Among grade ≥3 adverse reactions, MMAE and DM4 are primarily hematological toxicities (>5%), differing from other toxins, while thrombocytopenia is >5% across all toxins, and DM1 shows particularly prominent hepatotoxicity (7.2%), with peripheral neurotoxicity being more pronounced in MMAE (6.5%), while ocular toxicity is evident in MMAF, with lower incidence rates for DM1 and DM4.
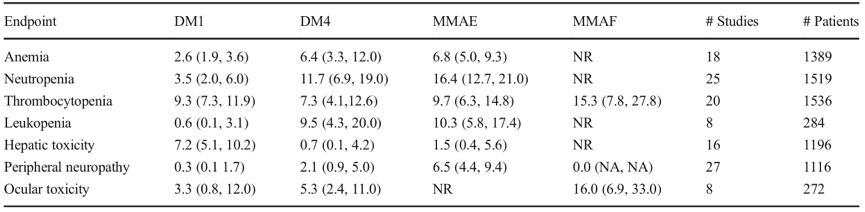
Table 1. Analysis of G3/4 adverse reactions, estimated values (95% confidence interval) NA=Not Applicable, NR=Not Reported
Among the 15 ADCs that have been approved, the most common grade ≥3 serious adverse events are hematological toxicities, including neutropenia, thrombocytopenia, leukopenia, and anemia. Off-target toxicities such as hematological toxicity, hepatotoxicity, and gastrointestinal toxicity may be related to the premature release of toxins into the bloodstream, non-tumor tissues, or tumor microenvironments, as well as the subsequent effects of toxins on healthy tissues. Recently, the pulmonary toxicity associated with DS-8201 has received widespread attention, and its pathogenesis is not yet fully understood. Current research indicates that it may be related to HER2 expression in pulmonary epithelial cells leading to on-target/off-tumor toxicity, and the non-target-specific endocytosis of ADC drugs by pulmonary macrophages may also be related. The two major technical challenges in ADC drug development are, first, the instability of the linkers with natural amino acids such as lysine or cysteine, which easily leads to off-target toxicity; second, the numerous amino acid sites on antibodies make it impossible to control the sites of linker attachment. Based on this, Ambrx has spent years developing patented unnatural amino acid site-specific conjugation technology to generate low-toxicity, high-stability, and uniform DAR ADC drugs.
To reduce the toxicity caused by the instability of ADC molecules, some ADC companies have adopted relatively weaker toxin molecules; while New Code Biology and its partner Ambrx aimed to achieve an efficient ADC drug through patented unnatural amino acid technology that is uniform in quality, stable in vivo, and ensures that released toxin molecules do not enter normal tissues to cause unknown toxicity when initially designing the anti-HER2 ADC. The most notable feature of the unnatural amino acid ADC technology platform is that it uses site-specific conjugation of unnatural amino acids, achieving stable attachment of two toxin molecules on each antibody molecule, with good uniformity and stable DAR values. The linker design considers the balance of hydrophobic/hydrophilic properties, using a unique non-cleavable linker to ensure that the toxin molecules do not fall off before entering the targeted tumor cells through antibody-targeted recognition, maximizing ADC endocytosis and minimizing off-target toxicity. Furthermore, due to the unique conjugation method of unnatural amino acids that does not disrupt the natural structure of the antibody, it preserves the natural conformation of the antibody, increasing metabolic stability, allowing it to achieve similar or even higher efficacy than other ADCs at lower dosing levels. In terms of cytotoxins, MMAF analogs are used, which have strong anti-tumor activity, short in vivo half-life, and extremely low concentrations of free toxins in the body, further achieving precise tumor killing and reducing off-target toxicity.
ARX788 is conjugated by a fully humanized HER2 monoclonal antibody and the microtubule inhibitor AS269, with a DAR of 2 at the optimal positions of the two heavy chains of the HER2 monoclonal antibody (pAF1221 and pAF114), where the ketone functional group on pAF specifically binds to the hydroxyl group of the microtubule inhibitor AS269 to form an oxime bond. The pAF-oxime bond is extremely stable and non-cleavable, and the only metabolic product in vivo is pAF-AS269, with no free toxin AS269, greatly improving efficacy and safety. Preclinical studies show that ARX788, with a DAR of 2, has higher anti-tumor activity compared to traditional cysteine-conjugated ADCs with a DAR of 4.5. ARX788 is highly stable in vivo, with serum concentrations of total antibodies and ADCs remaining essentially consistent, and very low concentrations of free toxins in the serum, significantly reducing systemic toxicity caused by toxins. The phase I clinical study led by our hospital on ARX788 monotherapy for HER2-positive advanced breast cancer showed that in the 1.5mg/kg RP2D dosage group with 29 subjects, the ORR was 65.5%, the DCR was 100%, and the median PFS was 17.02 months, with manageable safety, where most AEs were grade 1-2, and no DLT or drug-related deaths occurred, with the main AEs being liver, lung, and ocular adverse reactions. Similarly, the interim analysis of the phase II/III clinical study led by our hospital on ARX788 monotherapy for HER2-positive advanced breast cancer reached the efficacy threshold set by the protocol, with the incidence of pulmonary and ocular toxicity being higher, while the common adverse events were significantly lower than those of similar drugs, indicating that ADC drugs based on the unnatural amino acid site-specific conjugation technology platform have significant efficacy and safety advantages.
ARX517 is another ADC drug targeting PSMA based on the same unnatural amino acid technology platform, which also uses the non-cleavable linker and AS269 toxin. At the recently held ESMO conference, Ambrx announced preliminary data from its phase I/II clinical trial APEX-01 for metastatic castration-resistant prostate cancer (mCRPC), showing that ARX517 monotherapy demonstrated ideal safety and encouraging anti-tumor efficacy. The PK curves of total antibodies and ADCs at all dose levels of ARX517 are almost overlapping (Figure 1), with a half-life of 6-10 days, and very low serum concentrations of pAF-AS269, with a Cmax of 0.02–0.2 ng/mL (Figure 2), showing significant stability advantages and drug exposure duration compared to traditional lysine and cysteine conjugation technologies, with significantly reduced off-target toxicity.
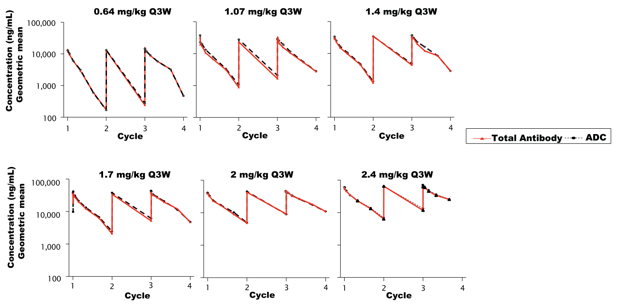
Figure 1. The PK curves of total antibodies and ADCs in all dose groups are nearly overlapping, indicating strong stability
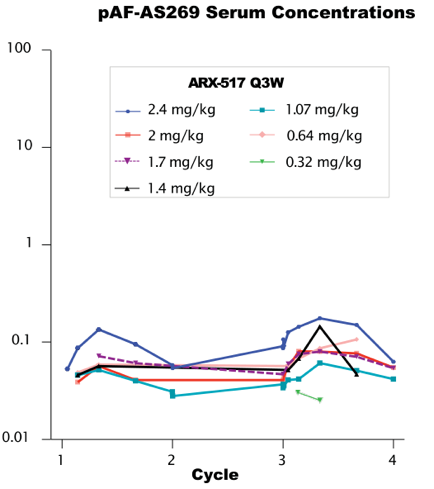
Figure 2. Low levels of free toxins observed in all dose groups
The study enrolled 65 PSMA-unselected prostate cancer subjects, with dose groups of 2.0-2.88 mg/kg (cohorts 6-8), a median of 4 lines of treatment (maximum 13), and 52% (12/23) of subjects had PSA declines of over 50%, with a clear dose-dependent relationship in PSA decline (Figure 3);

Figure 3. PSA decline shows a dose-dependent relationship
50% (3/6) of subjects who had previously received PSMA-TRT treatment had PSA declines of over 50%; consistent with PSA performance, 81% (17/21) of subjects had ct-DNA declines of over 50%. In the 1.4mg/kg-2.88mg/kg (cohorts 4-8) dose group, 56% (5/9) of subjects had tumor shrinkage, and at doses of 2.0-2.8mg/kg, 50% (2/4) of subjects had target lesions shrink by over 30%. No drug-related DLTs and SAEs occurred in all dose groups, with common AEs including dry mouth, dry eyes, fatigue, and diarrhea, while the incidence of hematological toxicity and liver and kidney toxicity was low, with no pulmonary toxicity or severe ocular toxicity. The incidence of grade 3 drug-related AEs was less than 10%, with no grade 4 or 5 AEs, and the drug-related treatment discontinuation rate was only 3.1%, with plans to increase to 3.4mg/kg for continued observation. Similarly, another ADC drug targeting CD70 based on the unnatural amino acid site-specific conjugation technology, ARX305, has reached a climbing dose of 1.3mg/kg, showing good safety, with no severe drug-related toxicity except for lymphocyte reduction related to CD70 targeting, and multiple subjects had tumor shrinkage.
ADC drugs based on the unnatural amino acid site-specific conjugation technology platform can maximize the balance between efficacy, human tolerance, and levels of cytotoxicity. They currently appear promising in addressing issues of ADC stability, safety, and druggability. New Code Biology has developed a new generation of unnatural amino acid technology platform over more than five years and is about to launch newly designed ADC products.
Expert Introduction
Fudan University Affiliated Tumor Hospital, Head of Phase I Clinical Research Ward, Chief Physician of Oncology, Shanghai “Medical Star” Outstanding Talent Award Recipient, Shanghai Anti-Cancer Association Oncology Drug Clinical Research Professional Committee, Incoming Chairman, Chinese Anti-Cancer Association Breast Cancer Professional Committee, Standing Committee Member, Chinese Anti-Cancer Association Breast Cancer Professional Committee Youth Committee, Deputy Convener, Yangtze River Academic Breast Alliance YBCSG, Chairman, Chinese Research Hospital Association Breast Professional Committee Youth Committee, Deputy Chairman, National Anti-Cancer Drug Clinical Application Monitoring Youth Committee, Deputy Chairman, Shanghai Anti-Cancer Association Oncology Cardiology Professional Committee, Deputy Chairman, CSCO Oncology Support and Rehabilitation Treatment Expert Committee, Standing Committee Member of Chinese Rehabilitation Medical Association Oncology Rehabilitation Professional Committee, Standing Committee Member of CSCO Breast Cancer Expert Committee, Member of Chinese Anti-Cancer Association Oncology Clinical Research Management Professional Committee, Member of the National Medical Products Administration CDE’s First Batch of Clinical Part-time Reviewers, Awarded 2023’s Top Ten Medical Pioneers and 2023 “People’s Good Doctor” Outstanding Contribution Award, Associate Editor of “Diseases & Research”
First/Total/Corresponding SCI publications 70 articles (Lancet Oncol, Ann Oncol, Nat Commun, Clin Cancer Res, J Hematol Oncol, etc.)
1. Maecker H, Jonnalagadda V, Bhakta S, et al. Exploration of the antibody–drug conjugate clinical landscape[J/OL]. mAbs, 2023, 15(1). https://doi.org/10.1080/19420862.2023.2229101.
2. Colombo R, Rich J R. The therapeutic window of antibody drug conjugates: A dogma in need of revision[J/OL]. Cancer Cell, 2022, 40(11): 1255-1263. https://doi.org/10.1016/j.ccell.2022.09.016.3. Dumontet C, Reichert J M, Senter P D, et al. Antibody–drug conjugates come of age in oncology[J/OL]. Nature Reviews Drug Discovery, 2023, 22(8): 641-661. https://doi.org/10.1038/s41573-023-00709-2.4. Tarantino P, Ricciuti B, Pradhan S M, et al. Optimizing the safety of antibody–drug conjugates for patients with solid tumours[J/OL]. Nature Reviews Clinical Oncology, 2023, 20(8): 558-576. https://doi.org/10.1038/s41571-023-00783-w.5. Joanna C Masters, Dana J Nickens, Dawei Xuan, et al. Clinical toxicity of antibody drug conjugates: a meta-analysis of payloads. Invest New Drugs, 2018, 36(1):121-135. https://doi.org/10.1007/s10637-017-0520-6.6. APEX-01: First-in-human phase 1/2 study of ARX517, an anti- prostate-specific membrane antigen (PSMA) antibody-drug conjugate (ADC), in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC).ESMO 2023. 1804PZhang J, Ji D, Shen W, Xiao Q, Gu Y, O’Shaughnessy J, Hu X. Phase I Trial of a Novel Anti-HER2 Antibody-Drug Conjugate, ARX788, for the Treatment of HER2-Positive Metastatic Breast Cancer. Clin Cancer Res. 2022 Jun 29:OF1-OF10. https://doi: 10.1158/1078-0432.CCR-22-0456.