ADC Field Blooms, new technologies evolve like a rolling tide, propelling biomedicine into high-speed mode. This is the best of times. As early as the early 20th century, Paul Ehrlich first proposed the concept of “magic bullets”; however, the first ADC drug, Mylotarg® (gemtuzumab ozogamicin), was not approved until nearly a century later. Every cloud has a silver lining, as of July 2022, a total of 14 ADC drugs have been approved globally for hematologic malignancies and solid tumors[1]. Additionally, there are currently over 100 ADC candidates in various stages of clinical trials. With the continuous expansion of targets and indications, ADCs are leading a new era of targeted cancer therapy, potentially transforming the future landscape of targeted drug treatment[2].
Figure 1 ADC Development Milestones[1]
Among the 14 approved ADC drugs, those with payloads of MMAE or MMAF account for 6, nearly 50%. The linker for protease B cleavable VC ADC drugs also has 6, accounting for nearly 50%. At the same time, there are over 40 ongoing pipelines with MMAE as the payload, and protease cleavable VC ranks first in the number of active ADC drug pipelines under research[3].
Table 1: Linker and Payload Technical Characteristics of the 14 Approved ADCs

MMAE/MMAF Sources and Binding Modes
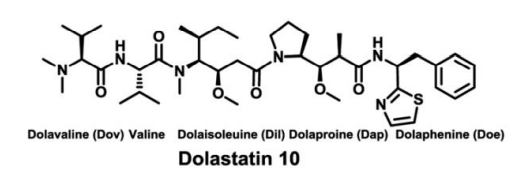
MMAE/MMAF are auristatin analogs; auristatin E was designed in 1992 by researchers at Arizona State University based on the structure of the natural cytotoxin dolastatin 10 (from the sea hare). Dolastatin 10 was isolated from the marine mollusk [6].
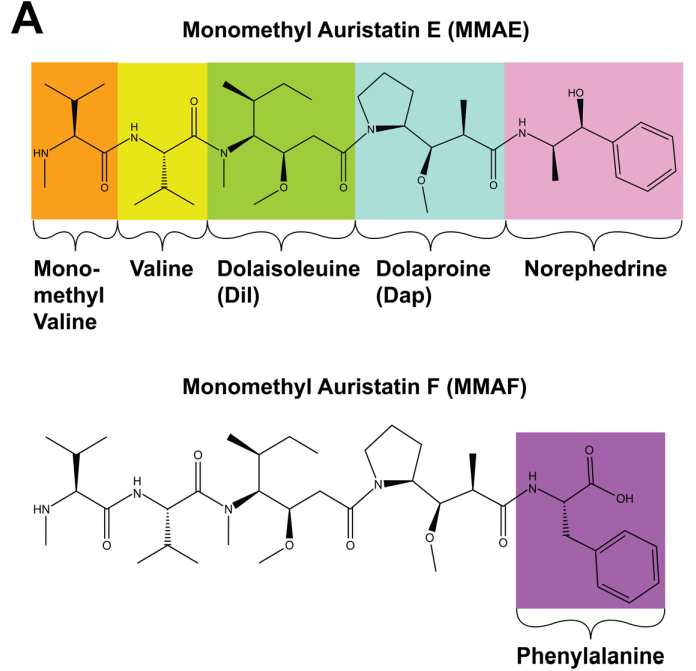
MMAE is a peptide composed of five units: three unnatural amino acids Dolavaline (Dov), dolaisoleuine (Dil), dolaproine (Dap), one natural amino acid Valine, and a C-terminal Norephedrine [6].
Figure 2 Structure of Dolastatin 10 and MMAE/MMAF[7][8]
Microtubules are the basic units that make up microtubules. Microtubules are heterodimers formed by the polymerization of α- and β-tubulin molecules and are highly dynamic[8]. MMAE/MMAF inhibit cell division by blocking the polymerization of tubulin, achieving anti-tumor effects. These molecules are highly stable, showing no signs of degradation in plasma, liver lysosomal extracts, or proteases like cathepsin B. In some clinical trials for lymphoma, leukemia, and solid tumors, MMAE has also demonstrated strong activity[9].
To date, most auristatin-based ADCs use one of the two auristatins, namely MMAE and MMAF. Due to their C-terminal modifications, these two compounds have different characteristics and allow for different conjugation strategies for monoclonal antibodies. Anionic carboxyl groups prevent MMAF from escaping cancer cells through passive diffusion across the cell membrane, thus limiting bystander effects.
Figure 3 Binding Sites of Tubulin and MMAE/MMAF[7]
The MMAE molecule (blue) binds at the interface between the β-tubulin subunit (light gray) and the α-tubulin subunit (dark gray), but has broader contact with the β-tubulin subunit. The binding constant (KD) value for FITC-MMAE is 291 nM, while that for FITC-MMAF is 63 nM, indicating that MMAF has a stronger binding affinity for tubulin than MMAE[7][8].
MMAE/MMAF and Their Linker Patent Status
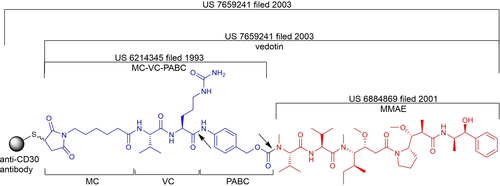
In 1993, scientists at BMS filed a patent describing a linker composed of PABC-VC cleavable by cathepsin B (US 6214345; Figure 5). In 2001, scientists at Seattle Genetics designed a derivative of auristatin E called monomethyl auristatin E (MMAE; US 6884869)[4].
Figure 4 Patent Status of MC-VC-PABC-MMAE[4]
In 2003, Seattle Genetics submitted a patent describing the conjugation of MC-VC-PABC with MMAE; this linker-cell payload was named vedotin (US 7659241; Figure 5). This patent also described an ADC targeting CD30.
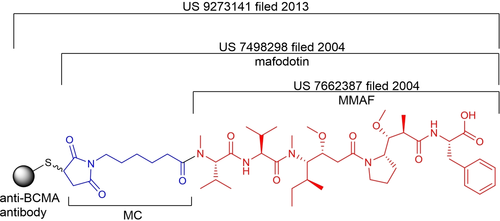
In 2004, scientists at Seattle Genetics designed an auristatin F analog with a monomethylated amino part linked to a linker (US 7662387). The MC linked to MMAF produces MC-MMAF, also known as mafodotin (US 7498298). Subsequently, various MMAF analogs were described in a 2007 patent, including MMAF derivatives with two carboxyl groups (US 7745394). In 2009, GlaxoSmithKline signed a licensing agreement with Seattle Genetics to utilize their linker-payload technology.
Figure 5 Patent Status of MC-MMAF[4]
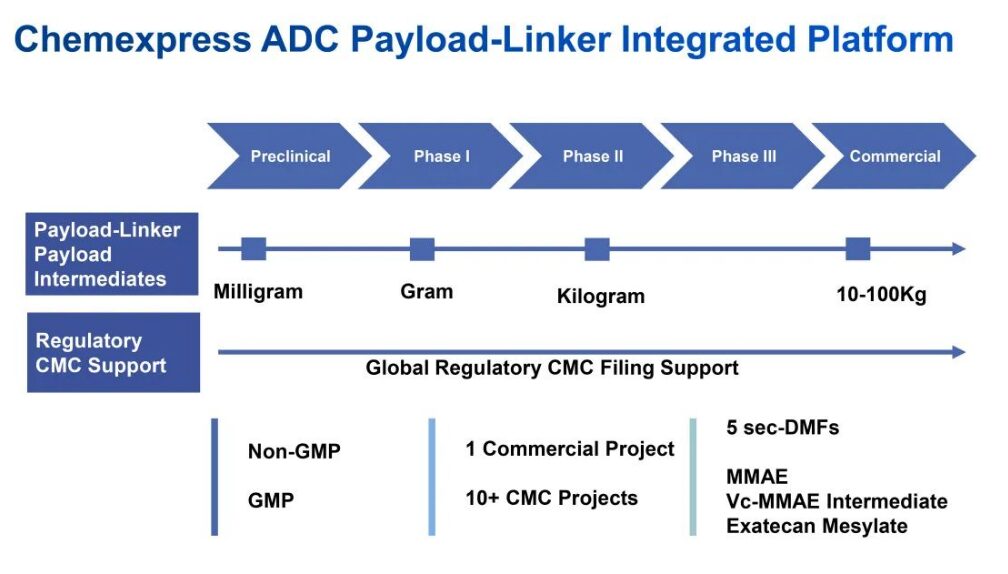
Haoyuan Pharmaceutical has nearly 70 Payload-Linker CDMO clients, with project stages covering preclinical, clinical I/II/III, and market stages. Among them, nearly 40 clients are in the Auristatin category, with cooperative products including MMAE, Vc-MMAE, MMAF, etc. There are DMF filings for high-level intermediates including Vc-MMAE[3].
As of July 2022, Haoyuan Pharmaceutical has registered five small molecule products related to ADC drugs with the US FDA DMF, including:
— MMAE CAS#474645-27-7 (DMF code: MF036741);
— MMAE First High-Level Intermediate CAS#160800-65-7 (DMF code: MF035548);
— MMAE Second High-Level Intermediate CAS#863971-44-2 (DMF code: MF035549);
— Vc-MMAE Linker High-Level Intermediate CAS#159857-80-4 (DMF code: MF035550);
— Exatecan Mesylate CAS#169869-90-3 (DMF code: MF036708);
Based on the analysis of the 14 approved ADC drugs, those using MC-Val-Cit-PABC, MMAE/MMAF as linkers and payloads are currently the most commonly used, most successful, and highest drug-like combination. For innovative pharmaceutical companies, choosing suppliers with DMF filings and directly referencing their DMF filing data during application can save a lot of manpower and communication costs, thus accelerating the IND application approval process.
The US DMF (Drug Master File) guidelines are archival materials submitted to the FDA (the global authority on drug regulation) for review, implemented since 1989 and still in use today. DMF includes confidential details regarding the facilities, operating processes, and materials used during the production, operation, packaging, and storage of products.
About Haoyuan Pharmaceutical
Shanghai Haoyuan Pharmaceutical Co., Ltd. (hereinafter referred to as “Haoyuan Pharmaceutical”) is one of the earliest companies in China to conduct ADC Payload-Linker research. It has developed a series of cutting-edge highly active toxins and constructed a diverse Payload-Linker library, primarily providing CMC and CDMO services related to the small molecule chemical components of ADC drugs, covering all aspects of toxin (Payload), linker (Linker), and payload-linker (Payload-Linker) development, process optimization, process validation, registration application, and GMP industrialization.
For a long time, Haoyuan Pharmaceutical has served the R&D and production of ADC drugs both domestically and internationally, possessing strong product and intermediate development capabilities, a wide variety of inventory, large-scale production capacity, fast supply in the industry, and high cost-effectiveness. Currently, it has accumulated experience in the synthesis of over 500 small molecules related to ADCs, assisting clients in completing multiple Payload-Linker CMC services and clinical registration applications, efficiently and qualitatively providing professional CDMO services to clients.
[1] New Technologies Bloom Together for Bettering Cancer Drug Conjugates. Pharmacol Rev 74:680–711, July 2022
[2] Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduction and Targeted Therapy (2022) 7:93
[3] Service Introduction | Haoyuan Pharmaceutical Focuses on Payload-Linker CDMO Services, Safeguarding the Most ADC R&D Pipelines with MMAE. Haoyuan Pharmaceutical. 2022.06.02
[4] A Patent Review on FDA-Approved Antibody-Drug Conjugates, Their Linkers and Drug Payloads. ChemMedChem 2022, e202200032 (1 of 11)
[5] “Magic Bullets” ADC. Haoyuan Pharmaceutical 2021.11.24
[6] Drugs and cosmetics from the sea. Mar. Drugs 2004, 2(2), 73-82
[7] Structural Basis of Microtubule Destabilization by Potent Auristatin Anti-Mitotics. PLOS ONE, August 12, 2016
[8] Cytotoxic Payloads for Antibody–Drug Conjugates. The Royal Society of Chemistry 2019
[9] Anti-CD22 and anti-CD79B antibody drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukemia, 2015, 29(7): 1578-86.
[10] Congratulations! Haoyuan Pharmaceutical MMAE Obtained US FDA DMF Filing. Haoyuan Pharmaceutical 2022.07.28
Disclaimer: The publication/reproduction of this article is solely for the purpose of disseminating information and does not imply endorsement of the views of this public account or verification of the authenticity of its content. Any judgments made based on this content are at your own risk.If there is any infringement, please inform us and we will delete it!
Long press to follow this public account
Fan Group/Submission/Authorization/Advertisementetc.
Please contact the public account assistant
If you think this article is good, please click here↓