
Introduction
Antibodies are one of the key components of ADC drugs, serving as precise guiding components with the functions of targeting and toxin delivery. They can specifically recognize the target antigens on the surface of tumor cells, delivering toxins to the tumor cells and mediating the localization and endocytosis of ADCs in tumor cells[1].
After the ADC-antigen complex is internalized by tumor cells, the linker breaks or the antibody is digested within endosomes or lysosomes, releasing cytotoxic drugs that damage DNA or inhibit the synthesis of tubulin proteins, leading to cell death and exerting a bystander effect, damaging adjacent tumor cells.
(Previous Review: ADC In-Depth Series – Targeting)
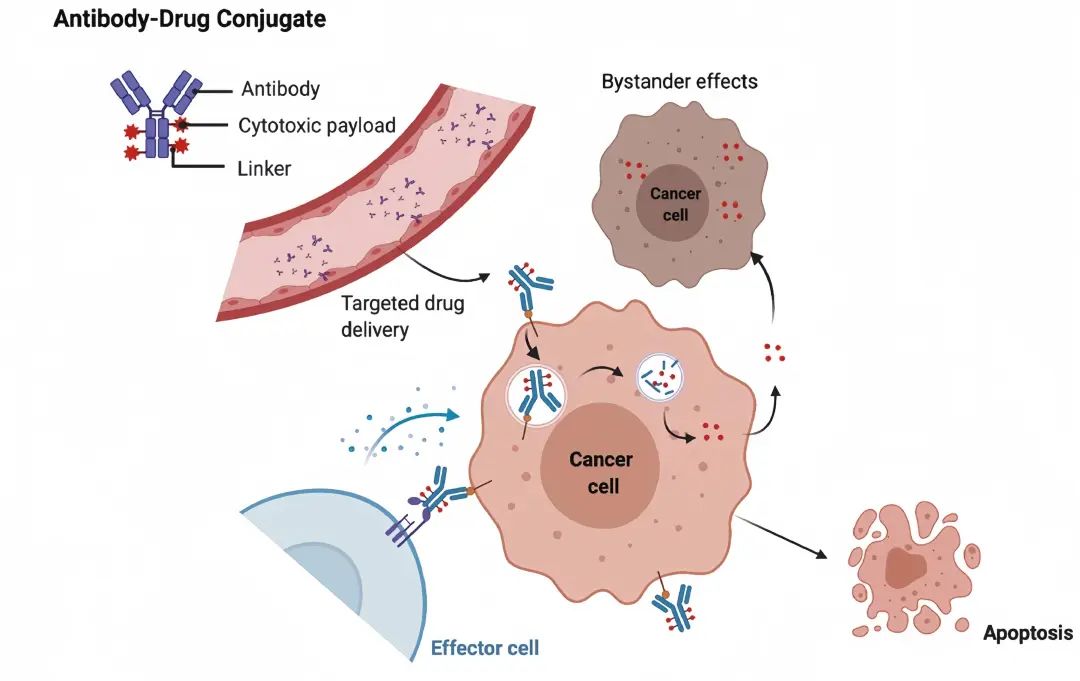
The Role of Antibodies in ADC Drugs[1]
Selection of Antibodies in ADC Drugs
In the early stages of ADC drug development, murine antibodies were primarily used, but due to severe immunogenic side effects, the failure rate was high. With the advent of recombinant technology, murine antibodies have largely been replaced by chimeric antibodies and humanized antibodies. Currently, an increasing number of ADCs use fully humanized antibodies with significantly reduced immunogenicity. Among the 14 ADC drugs approved globally, only brentuximab vedotin uses a chimeric antibody[1].
Humanized IgG is most commonly used in cancer immunotherapy and is further divided into IgG1, IgG2, IgG3, and IgG4 based on differences in the heavy chain region and hinge region. Different structural variations lead to different affinities and half-lives for these IgGs. IgG1, IgG2, and IgG4 have the same half-life, but IgG2 and IgG4 have lower affinities for immune effector cells. IgG3 has high affinity but a lower half-life[3]; IgG1 antibodies bind more efficiently to FcγR, inducing strong effector functions such as antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC)[1]. Therefore, most ADC drugs currently use IgG1 antibodies.
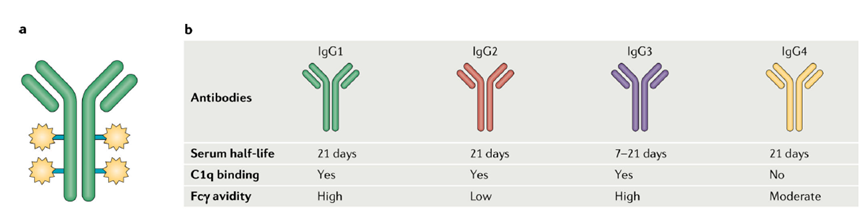
Affinity and Half-life of Different IgGs[3]
Ideal Antibodies in ADC Drugs
Novel Antibodies in ADC Drugs
1. Bispecific Antibodies
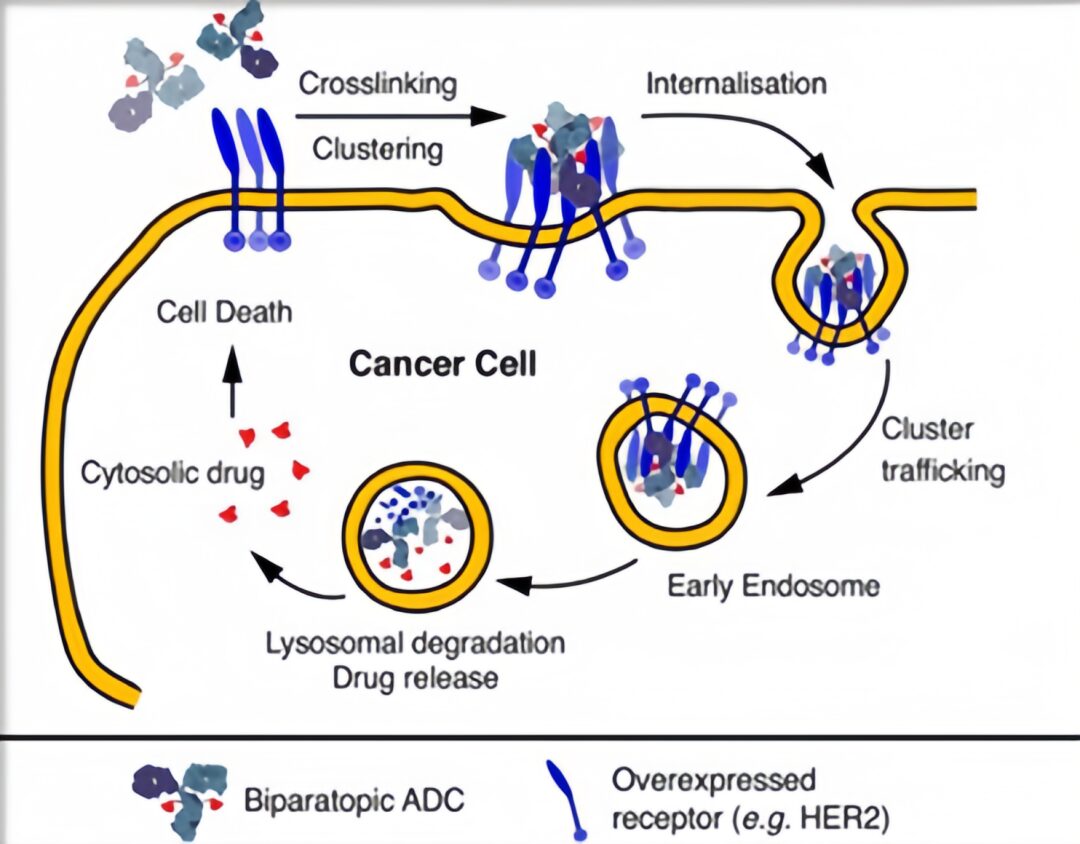
Dual-Epitope ADC
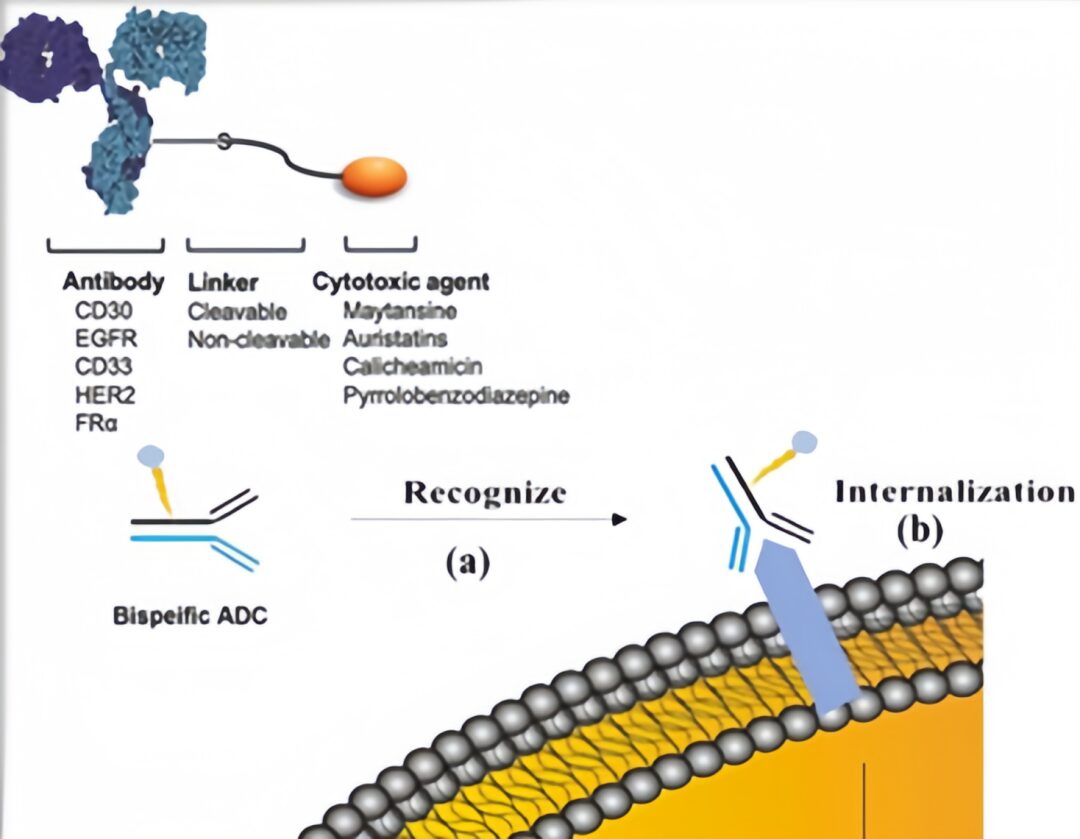
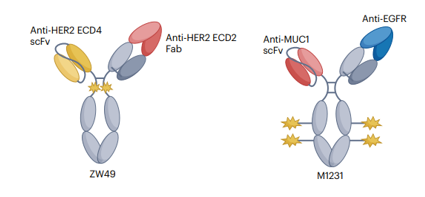
Dual-Target ADC
ZW-49 is a bispecific antibody ADC developed by Zymeworks, which can specifically bind to two non-overlapping epitopes of the HER2 receptor; M1231, co-developed by Sutro Biopharma and EMD Serono, is a bispecific antibody ADC targeting EGFR and MUC1.
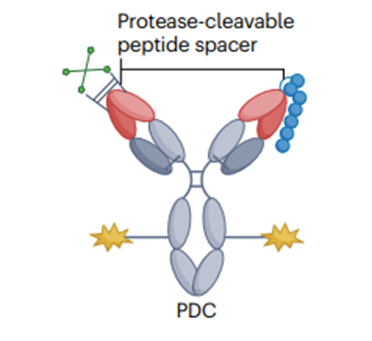
2. Pro-antibodies
Pro-antibody-drug conjugates are a new type of ADC drug with high specificity and low off-target toxicity. This technology utilizes linker-conjugated masking peptides at the light chain end of monoclonal antibodies, preventing the antibody from binding to the target protein, while corresponding protein kinases that can cleave the linker exist in the tumor microenvironment, allowing the antibody to bind to the target protein. The precursor antibody molecules of conditionally activated antibody pro-drug conjugates (PDC) are also IgG[4].
3. Antibody Fragments
Smaller drug carriers can help drugs better penetrate solid tumors, providing a wider therapeutic window. Using bivalent antibody fragments, antibody Fab fragments, single-chain antibodies (scFv), etc., with molecular weights ranging from 5 to 100 kDa as drug delivery vehicles shows great promise in the development of ADC drugs.
Conclusion
In conclusion, as interest in ADC technology grows, the development of research technologies will promote better optimization of each component that makes up ADC drugs. To ensure ADC drugs effectively exert their effects and achieve the expected efficacy and safety, their design and development should focus on the research of targets, antibodies, linkers, payloads, coupling methods, and their reasonable combinations, facilitating the iterative update of ADC products.
References:
1.Fu Z, Zhang Y. et al. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022;7(1):93.
2.Zeng Hongye, Ning Wenjing, Luo Wenxin. Research progress on the antibody composition and target action of ADC drugs*[J]. Chinese Journal of Biotechnology, 2022, 42(5): 69-80.
3.Drago, J.Z, et al. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat Rev Clin Oncol 18, 327–344 (2021).
4. Tsuchikama K, Anami Y, Ha SYY, Yamazaki CM. Exploring the next generation of antibody-drug conjugates. Nat Rev Clin Oncol. 2024 Mar;21(3):203-223..
* This article is intended to provide scientific information to medical professionals and does not represent the views of this platform.


