Skip to content
 After 2019, ADC drugs have been approved for marketing one after another, and the approval of Aidiqi in 2021 marked a milestone in the development of domestic ADC innovative drugs. The broad therapeutic prospects and huge market size have stimulated many pharmaceutical companies to ignite their enthusiasm for ADC drug research and development, leading the industry into a rapid development phase. Although the overall trend of development is progressive, the road is tortuous. With the rush of research, how to avoid product homogenization, break the cruel competitive landscape, and explore innovative development paths has become a top priority. The following discussion is based on the author’s rudimentary understanding, exploring why some popular targets for ADC drugs stand out, as well as the development status and challenges of some innovative targets, and finally looking forward to the future path of innovation.
Currently, the targets for ADC drug development include targeting tumor cell surface antigens and targeting tumor microenvironment antigens, among others. The main focus is on targeting tumor cell surface antigen proteins, which deliver effective payloads into cells by recognizing highly expressed specific markers, exerting cytotoxic effects.
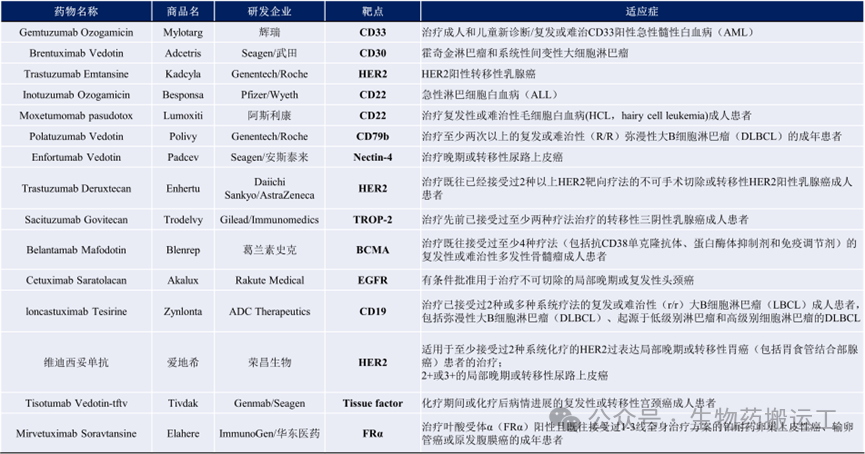
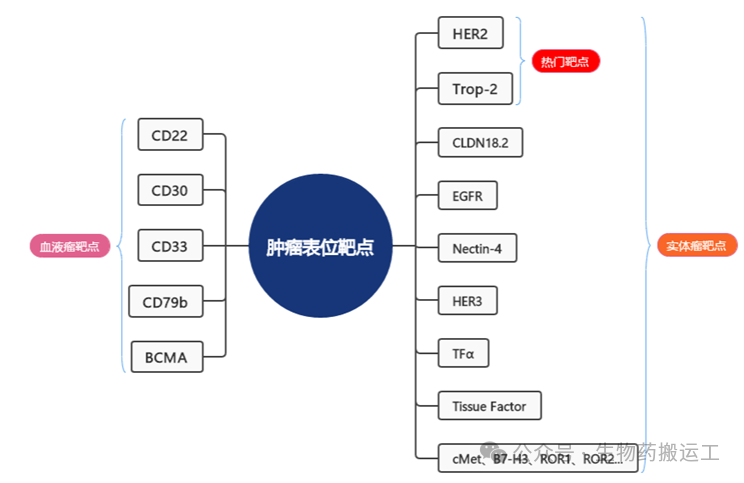
Tracing back to the 15 ADC drugs approved for marketing, there are a total of 11 targets, including CD33, CD30, HER2, CD22, CD79b, Nectin-4, BCMA, EGFR, CD19, Tissue Factor, and FRα (as shown in Figure 1). Among them, the early successful ADC drug targets mainly focus on hematological malignancies, such as CD22, CD30, CD33, CD79b, and BCMA. The popular targets in solid tumors, HER2 and Trop-2, have also been well validated in clinical settings. In addition, some emerging targets are attracting the interest of pharmaceutical companies both domestically and internationally, such as CLDN18.2, EGFR, Nectin-4, and HER3 (as shown in Figure 2).
Figure 1 Summary of Approved ADC Targets
Figure 2 Major Tumor Surface Targets
After 2019, ADC drugs have been approved for marketing one after another, and the approval of Aidiqi in 2021 marked a milestone in the development of domestic ADC innovative drugs. The broad therapeutic prospects and huge market size have stimulated many pharmaceutical companies to ignite their enthusiasm for ADC drug research and development, leading the industry into a rapid development phase. Although the overall trend of development is progressive, the road is tortuous. With the rush of research, how to avoid product homogenization, break the cruel competitive landscape, and explore innovative development paths has become a top priority. The following discussion is based on the author’s rudimentary understanding, exploring why some popular targets for ADC drugs stand out, as well as the development status and challenges of some innovative targets, and finally looking forward to the future path of innovation.
Currently, the targets for ADC drug development include targeting tumor cell surface antigens and targeting tumor microenvironment antigens, among others. The main focus is on targeting tumor cell surface antigen proteins, which deliver effective payloads into cells by recognizing highly expressed specific markers, exerting cytotoxic effects.
Tracing back to the 15 ADC drugs approved for marketing, there are a total of 11 targets, including CD33, CD30, HER2, CD22, CD79b, Nectin-4, BCMA, EGFR, CD19, Tissue Factor, and FRα (as shown in Figure 1). Among them, the early successful ADC drug targets mainly focus on hematological malignancies, such as CD22, CD30, CD33, CD79b, and BCMA. The popular targets in solid tumors, HER2 and Trop-2, have also been well validated in clinical settings. In addition, some emerging targets are attracting the interest of pharmaceutical companies both domestically and internationally, such as CLDN18.2, EGFR, Nectin-4, and HER3 (as shown in Figure 2).
Figure 1 Summary of Approved ADC Targets
Figure 2 Major Tumor Surface Targets
-
Sialic Acid-Binding Ig-Like Lectin 2 (CD22, Siglec-2) belongs to the immunoglobulin superfamily and the sialic acid-binding Ig-like lectin family. It is a type I transmembrane protein that can specifically bind to sialic acid (Sia)-containing glycans and inhibit B cell receptor (BCR) signaling through its immunoreceptor tyrosine inhibitory motif (ITIM), playing a role in maintaining humoral immune homeostasis. CD22 is primarily located on the cell membrane of mature B cells and is expressed in most B cell malignancies, including acute lymphoblastic leukemia (B-ALL), non-Hodgkin lymphoma (NHL), and hairy cell leukemia (HCL).
Given that CD22 is a specific antigen target on many tumor cell surfaces and has the characteristic of endocytosis, it has become one of the most developed targets. However, the glycosylation of CD22 epitopes severely affects the targeting binding capacity of antibody drugs, limiting the development of high-affinity antibodies. Currently, there are 12 ADC drugs targeting CD22 under research globally, mainly developed by foreign companies (as shown in Figure 3).
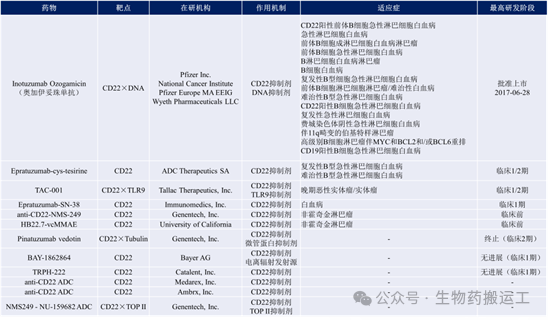 Figure 3 Summary of Anti-CD22 ADC Drugs
Figure 3 Summary of Anti-CD22 ADC Drugs
-
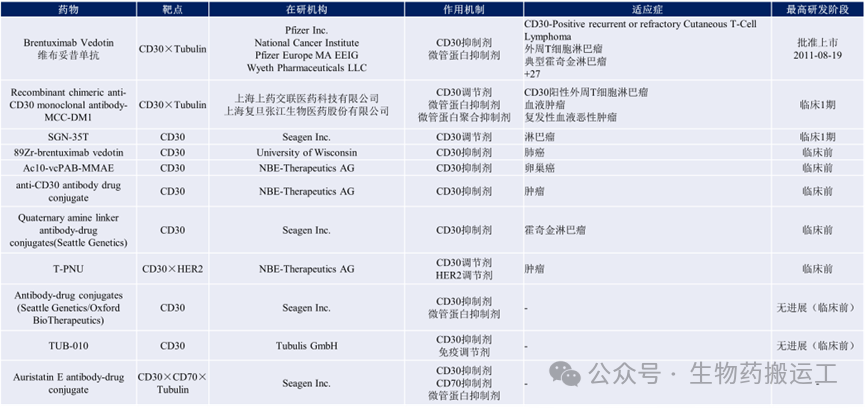
CD30 is a member of the tumor necrosis factor (TNF) receptor superfamily, which can promote cell proliferation or apoptosis by activating different signaling pathways. It is not expressed in healthy cells, has low expression on the surface of normally activated T and B cells, and is highly expressed on the surface of tumor cells (such as Hodgkin lymphoma and anaplastic large cell lymphoma). This differential expression makes CD30 an ideal target for tumor-targeting drug development. Although Brentuximab Vedotin has been approved for marketing, the drug development progress for this target has not been satisfactory, mainly focusing on the CAR-T field. Currently, apart from Brentuximab Vedotin, most are in phase 1 clinical and preclinical stages (as shown in Figure 4).
Figure 4 Summary of Anti-CD30 ADC Drugs
-
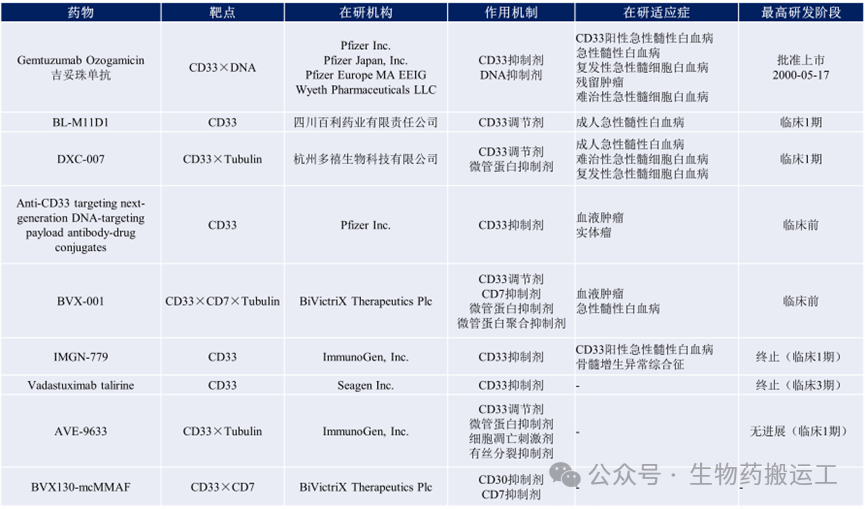
CD33 (Siglec-3), like CD22, belongs to the Siglecs family, is overexpressed in leukemia progenitor cells, as well as myeloid leukemia initiating cells, making it a potential target for AML (acute myeloid leukemia) immunotherapy. Similar to CD22, CD33 also has inhibitory characteristics on cell signaling cascades and endocytosis, but CD33 is not as conserved as CD22 and has various splice variants. Currently, there are 9 ADC projects targeting CD33 under research globally (as shown in Figure 5).
Figure 5 Summary of Anti-CD33 ADC Drugs
-
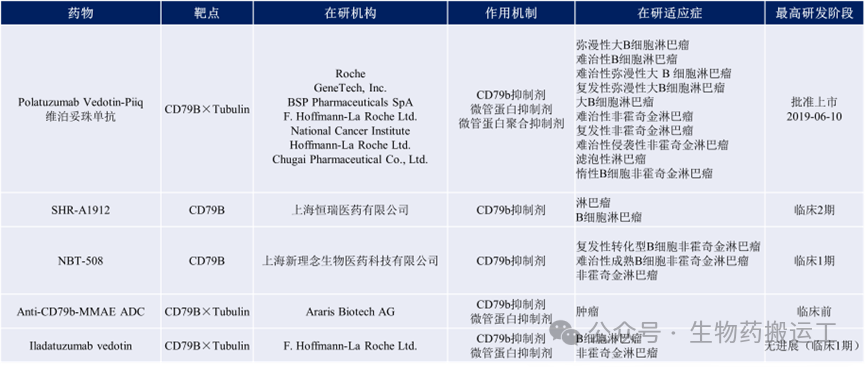
CD79B is a B cell surface antigen and a component of the BCR, expressed on almost all B cells, playing an important role in the expression and transport of BCR to the cell membrane. It is also highly expressed on various B cell NHL and chronic lymphocytic leukemia B cells, becoming one of the innovative targets for killing B cell tumors. Currently, there are 5 disclosed ADC projects targeting this target globally.
Figure 6 Summary of Anti-CD79B ADC Drugs
-
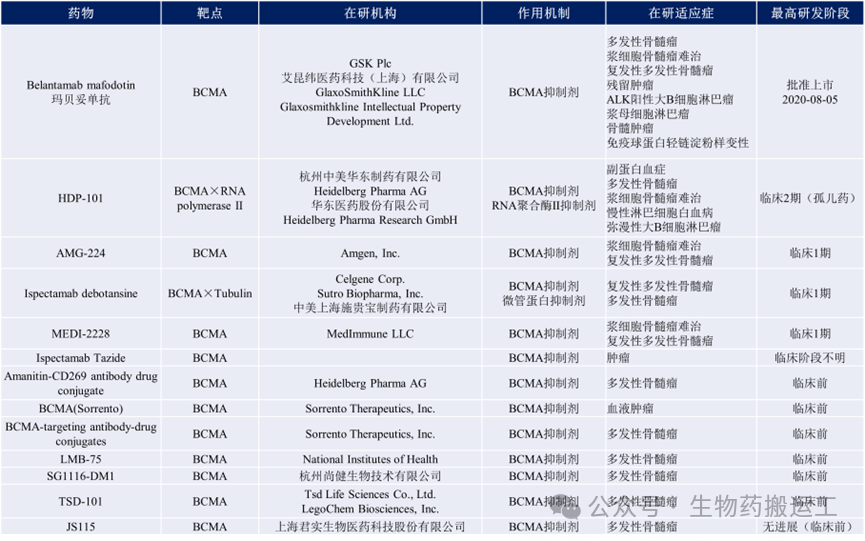
B Cell Maturation Antigen (BCMA) is a member of the tumor necrosis factor receptor superfamily (TNF-receptor), expressed on the surface of mature B cells and plasma cells, highly expressed in malignant proliferating B lymphocytes (e.g., myeloma cells, leukemia cells). Currently, there are 13 global ADC projects under research (as shown in Figure 7).
Figure 7 Summary of Anti-BCMA ADC Drugs
-
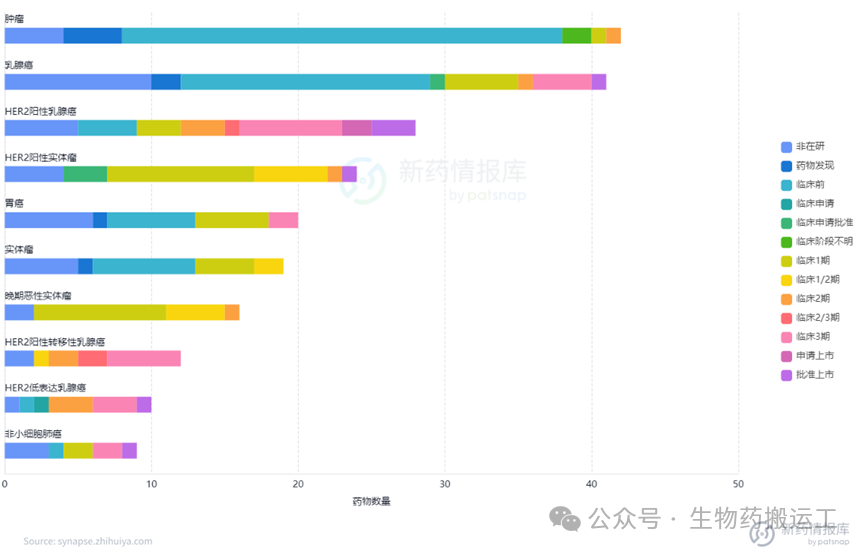
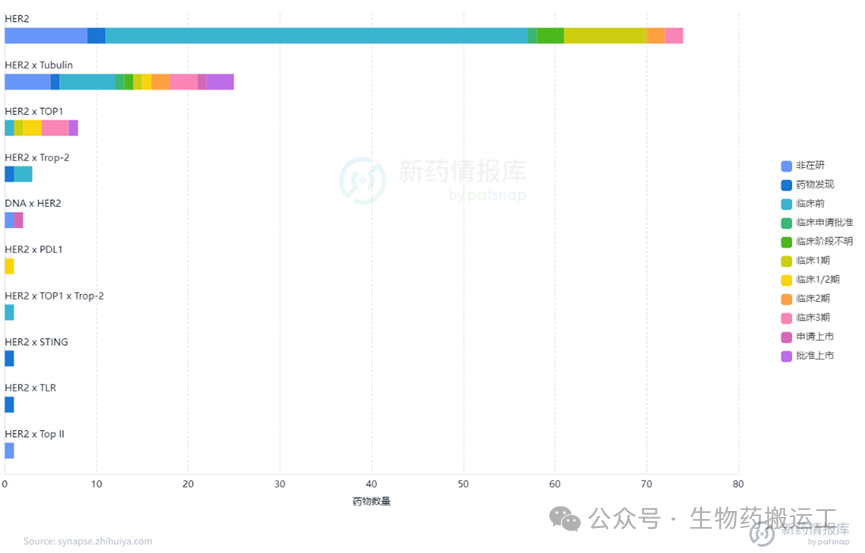
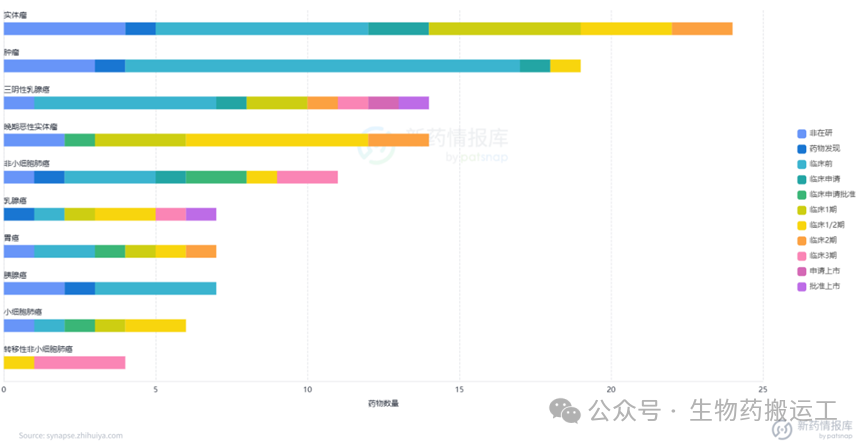
Human Epidermal Growth Factor Receptor-2 (HER2) is a member of the EGFR receptor tyrosine kinase family and is one of the most thoroughly studied genes in breast cancer to date. Overexpression of the HER2 gene leads to autophosphorylation of tyrosine residues in the cytoplasmic domain of heterodimers, triggering various signaling pathways that result in cell proliferation and tumorigenesis. From the disclosed information, as one of the most competitive targets, there are already 130 ADC projects targeting HER2. Among them, 4 have been approved for marketing, 2 have applied for marketing, and as many as 8 are in phase 3 clinical trials. The distribution of indications is also broad, commonly used for the treatment of breast cancer, solid tumors, gastric cancer, and other diseases (as shown in Figures 8-9). In addition to HER2 single-target ADCs, innovative designs of dual-target or multi-target ADCs combining HER2 with Trop-2/PDL1/TOP1/STING have also entered people’s vision (as shown in Figure 10).
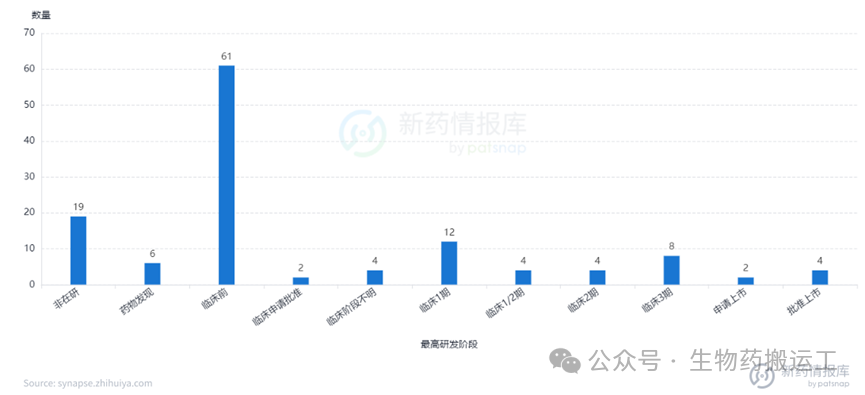 Figure 8 Highest Development Stage of HER2-ADC
Figure 9 Top Ten Indications for HER2-ADC
Figure 10 Top Ten Targets for HER2-ADC
Figure 8 Highest Development Stage of HER2-ADC
Figure 9 Top Ten Indications for HER2-ADC
Figure 10 Top Ten Targets for HER2-ADC
-
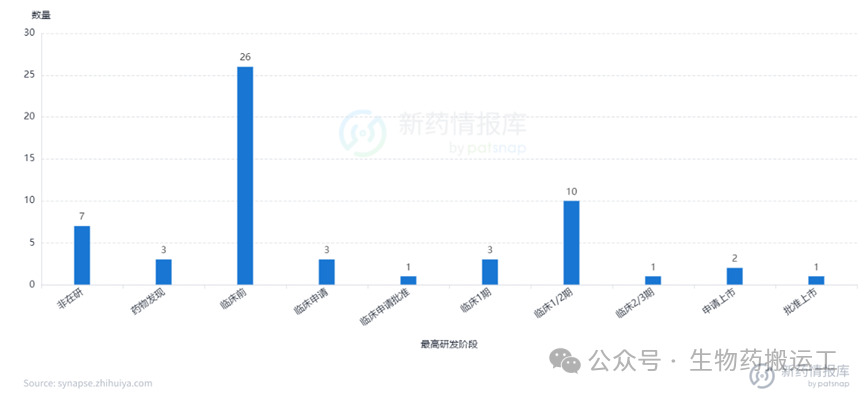
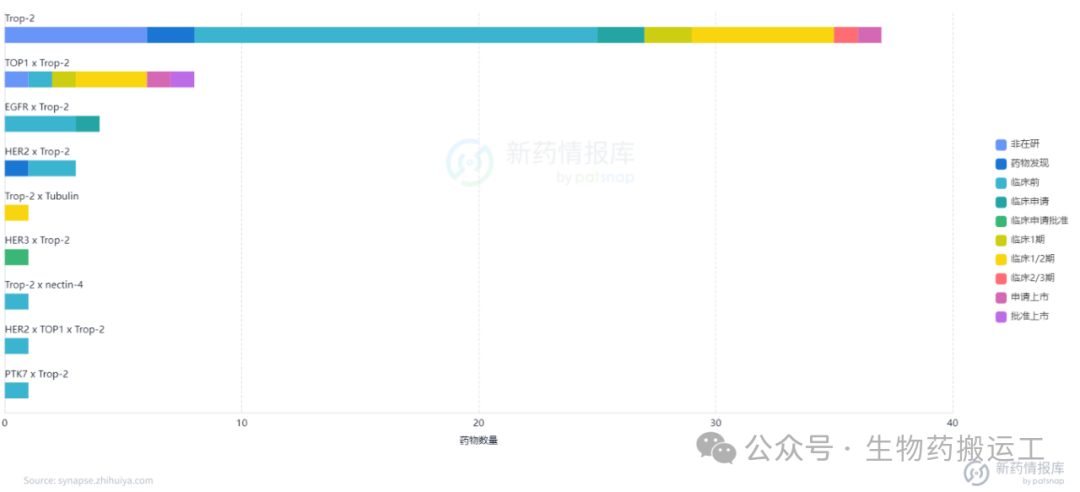
Human Trop2 (TROP2) is a cell surface transmembrane glycoprotein that mediates intracellular calcium signaling. TROP-2 is highly expressed in various human epithelial cancers, such as breast cancer, lung cancer, gastric cancer, and colorectal cancer. Currently, there are as many as 59 global ADC projects targeting TROP2 under research, among which Kelun-Biotech’s SKB264 has applied for marketing and is expected to become China’s first approved domestic innovative TROP2-ADC (as shown in Figures 11-13).
Figure 11 Highest Development Stage of TROP2-ADC
Figure 12 Top Ten Indications for TROP2-ADC
Figure 13 Top Ten Targets for TROP2-ADC
-
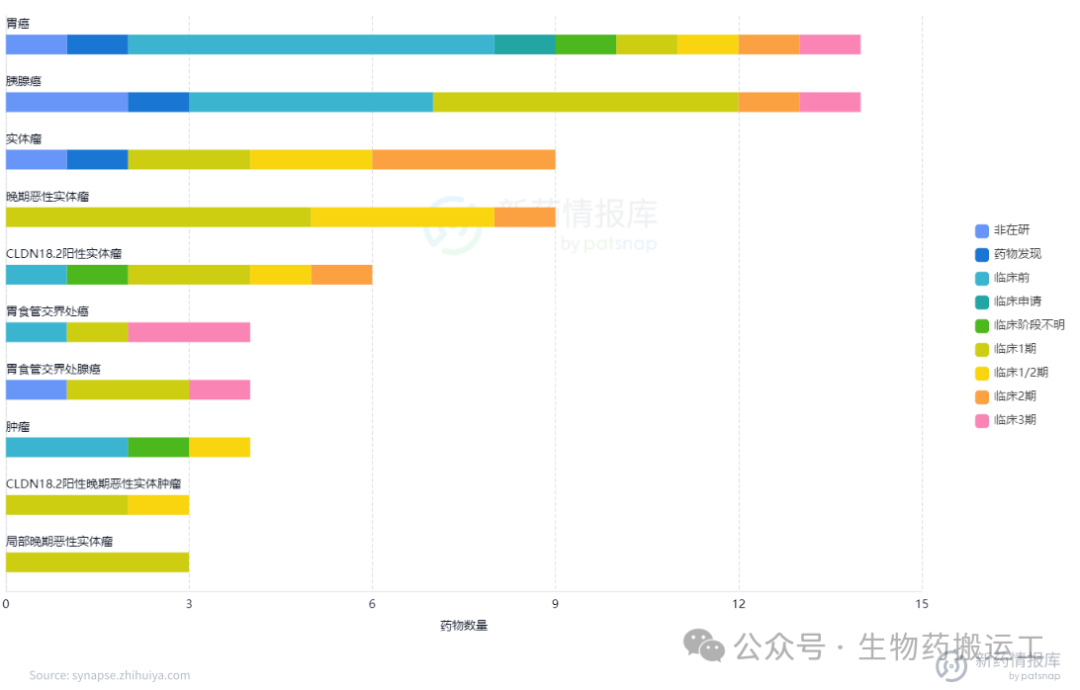
CLDN18.2 (Claudin18.2) is a stomach-specific membrane protein and is currently the most thoroughly studied Claudin family protein, mainly upregulated in gastric cancer and pancreatic cancer. The human CLDN18 gene can produce two protein isoforms, CLDN18.1 and CLDN18.2, which are highly expressed in lung and gastric tissues, respectively. Currently, there are 28 ADC projects under research targeting this target globally (Figures 14, 15), with domestic pharmaceutical companies having the most pipeline layouts, including Kanghua Biotech (CMG-901), Lixin Pharma (LM-302), and Innovent Biologics (IBI343), all of which have progressed to phase 3 clinical trials. From clinical results, ADCs targeting Claudin 18.2 have achieved significant progress. For example, the clinical results of CMG901 announced in 2023 showed that among 8 patients with Claudin 18.2 positive gastric cancer or gastroesophageal junction adenocarcinoma treated with CMG901, the objective response rate (ORR) was 75%, and the disease control rate (DCR) was 100%, with good safety and tolerability.
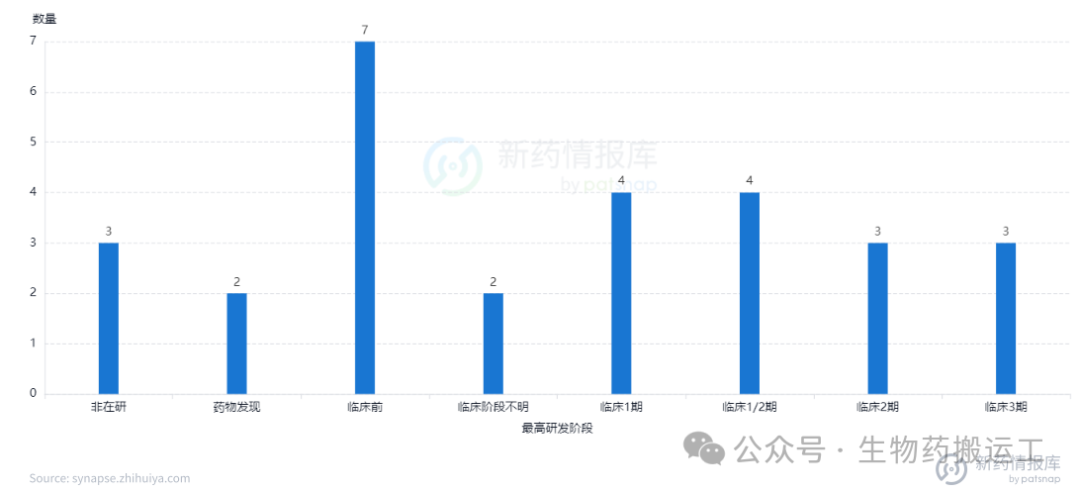 Figure 14 Highest Development Stage of CLDN18.2-ADC
Figure 15 Top Ten Indications for CLDN18.2-ADC
Tumor Microenvironment Targets
The tumor microenvironment (TME) is the environment in which tumors occur and grow, mainly composed of fibroblasts (CAFs), immune cells, adipocytes, vascular endothelial cells, and extracellular matrix (ECM) of tumor cells. By targeting the metabolism of microenvironmental cells, it can effectively promote anti-tumor immunity and influence tumor proliferation and metastasis. Therefore, tumor microenvironment (TME) targets have become a research hotspot, such as ADC targets acting on extracellular matrix components: fibronectin, tenascin-C, fibrin, and collagen IV, etc. However, currently, ADCs targeting tumor microenvironment targets have not achieved success in clinical settings, and there is still significant development space.
Figure 14 Highest Development Stage of CLDN18.2-ADC
Figure 15 Top Ten Indications for CLDN18.2-ADC
Tumor Microenvironment Targets
The tumor microenvironment (TME) is the environment in which tumors occur and grow, mainly composed of fibroblasts (CAFs), immune cells, adipocytes, vascular endothelial cells, and extracellular matrix (ECM) of tumor cells. By targeting the metabolism of microenvironmental cells, it can effectively promote anti-tumor immunity and influence tumor proliferation and metastasis. Therefore, tumor microenvironment (TME) targets have become a research hotspot, such as ADC targets acting on extracellular matrix components: fibronectin, tenascin-C, fibrin, and collagen IV, etc. However, currently, ADCs targeting tumor microenvironment targets have not achieved success in clinical settings, and there is still significant development space.
-
Carbonic Anhydrase IX (CA IX) is a type of metal enzyme that catalyzes the hydration of carbon dioxide to generate bicarbonate in hypoxic environments, overexpressed in more than 90% of renal clear cell carcinoma and almost not expressed in normal tissues. This characteristic makes CA IX an attractive target. However, there have been no positive results for ADC projects targeting this target. For example, although the BAY 79-4620 molecule showed good tumor-killing effects in in vitro efficacy tests, severe adverse reactions in clinical settings led to the termination of the study.
-
Fibroblast Activation Protein Alpha (FAPα) is a serine protease secreted by fibroblasts, involved in tissue remodeling and wound healing. Studies have found that monoclonal antibodies targeting FAPα have no anti-tumor activity, but significant anti-tumor effects were observed after conjugation with a toxin. According to disclosed information, there is currently only one ADC drug targeting this target under research, while there are 5 and 41 peptide conjugated drugs (PDC) and radionuclide conjugated drugs under research, respectively.
-
LRRC15 (leucine-rich repeat containing 15) is a leucine-rich repeat protein that is lowly expressed in normal tissues and highly expressed in various solid tumors such as breast cancer, osteosarcoma, and prostate cancer. Currently, only 1 ADC targeting this target is under research (Samrotamab vedotin), but the development is in phase 1 clinical trials with no progress.
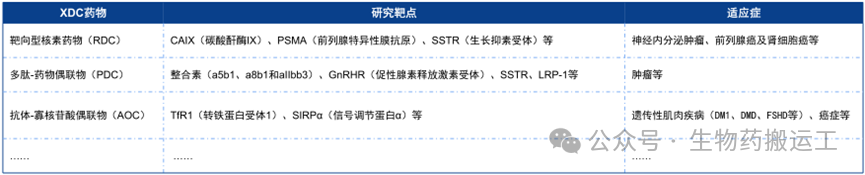
In addition to the above targets, some innovative targets also possess certain drug development potential, driving the development of XDC drugs, and the indications are no longer limited to tumor treatment (as shown in Figure 16).
Figure 16 XDC Drug Targets
-
Gonadotropin-Releasing Hormone Receptor (GnRHR) is a reproductive hormone that is synthesized and secreted by the hypothalamus and acts on the pituitary gland. The molecular weight of GnRH is 1.181 kDa, belonging to a secreted small molecular semi-antigen with very weak immunogenicity. By altering the molecular structure of GnRH and conjugating GnRH with large molecular carrier proteins, immunogenicity can be enhanced. Currently, there are only 3 peptide conjugated drugs under research targeting this target, and the development status is not optimistic. The fastest progress, Zoptarelin Doxorubicin, has already been terminated in phase 3 clinical trials due to severe adverse reactions and high mortality rates (structure shown in Figure 17), and the other 2 projects are also in a state of no progress.
Figure 17 Structure of Zoptarelin Doxorubicin
-
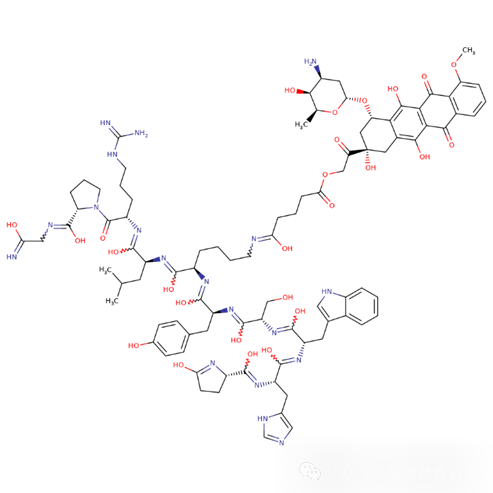
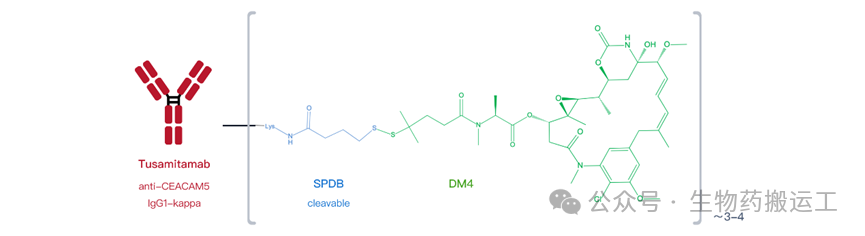
Carcinoembryonic Antigen Cell Adhesion Molecule 5 (CEACAM5) is one of the biological markers involved in tumor cell adhesion, differentiation, proliferation, and survival, and is overexpressed on the surface of various solid tumors. Currently, there are 7 ADCs targeting this target under research. The fastest progress is IBI-126 (ImmunoGen), which connects the tusamitamab antibody with a toxin via SPDB (as shown in Figure 18), but the clinical results of phase 3 announced in December 2023 showed that neither PFS nor OS reached the endpoint indicators.
Figure 18 Structure of Tusamitamab Ravtansine
The Future Path of Innovation
People have never ceased to explore and develop ADC targets, and the druggability of targets is also a challenge in ADC drug development. In addition to the specificity and abundance on the tumor surface, whether they have endocytosis is also an important criterion for evaluating targets. Compared to the currently hot development of endocytosis-type ADCs, the development of non-endocytosis-type ADCs is still relatively rare, but their clinical efficacy has been fully validated, providing new opportunities for future ADC development.
In addition to target selection, designing antibody structures based on target antigens is also another innovation point. Given the good serum half-life and strong immune activation ability, most antibody drugs currently use the IgG1 subtype, with only Mylotarg and Besponsa among the marketed ADCs using the IgG4 subtype for their antibody portions. In addition to monoclonal antibody forms, bispecific antibodies, antibody fragments, and nanobodies provide directions for the design of new XDC drugs. For example, the bispecific ADC targeting EGFR/HER3 (BL-B01D1) can kill EGFR-dependent tumors and reduce resistance caused by HER3, and the data in tumor models show that BL-B01D1 has better anti-cancer activity compared to HER3-ADC (U3-1402).
Ultimately, the innovation of targets requires a deeper understanding of disease occurrence, development, target distribution, and mechanism of action. Although the road ahead is long and arduous, the 21st century is the era of biology, and it is believed that more effective ADC drugs will emerge in the future, bringing hope for disease treatment.
[1] Huajin Securities Research Report 2023 “ADC Drugs: Innovating Forward, Setting Sail Overseas”
[2] New Drug Information Database https://synapse.zhihuiya.com/?csrfToken=
Scan the WeChat QR code to add the editor of the Bioproduct Circle, those who meet the conditions can join
Please indicate: Name + Research Direction!
All articles published by this public account are for the purpose of conveying more information, and the source and author are clearly indicated. Media or individuals who do not wish to be reproduced can contact us ([email protected]), and we will immediately delete it. All articles only represent the author’s views and do not represent the position of this site.