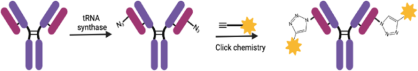
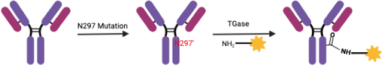
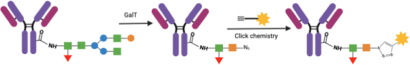
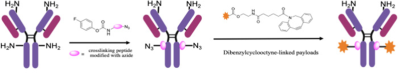

Conference Content
Time: September 22-23, 2023 (Friday-Saturday)
Location: Shanghai (hotel notification)
Guiding Unit: Shanghai Pharmaceutical Quality Association
Organizer: Four Leaf Clover Exhibition, Biological Products Circle
Co-organizer: Aoxing Group
Conference Fee: To provide conference benefits for quality experts in pharmaceutical companies, Registration FREE! (only a deposit of 50 yuan is required, which includes refreshments, conference materials, etc., and the deposit is non-refundable) (if meals are required, an additional 150 yuan will be charged for lunch for 2 days), first come first served, registration ends when full! Please secure your spot in advance!
Registration Method: Scan the QR code below or click on “Read Original” at the bottom of the article → Fill out the form → Successful registration (volunteers for registration, take on certain work, please consider carefully, no deposit required)!

After the organizing committee receives the registration information, they will conduct an initial screening based on the information and further communicate with the registrants for confirmation, achieving precise invitations. There will be an opportunity to enter the conference WeChat group (strict review required).