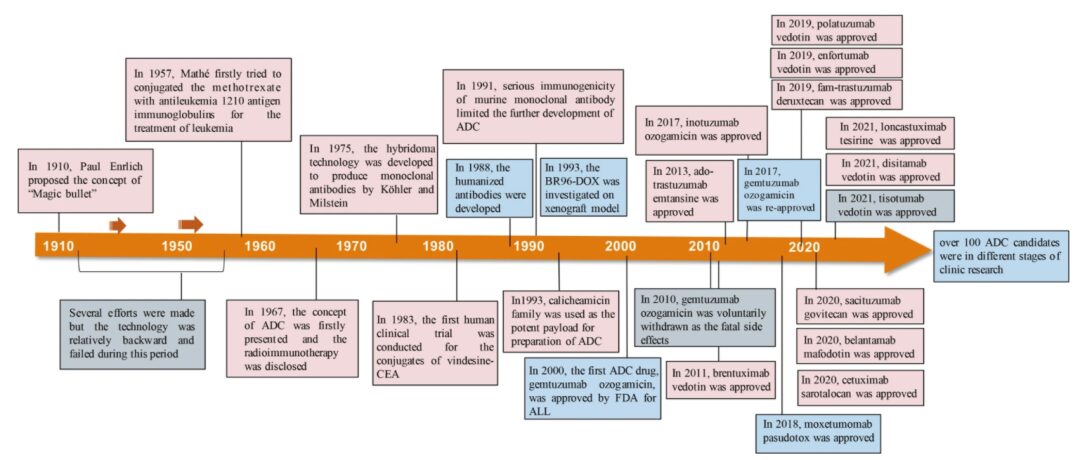
1
Pathogenesis and Treatment Status of Autoimmune Diseases
2
Can Immunomodulatory ADCs Become a New Weapon?
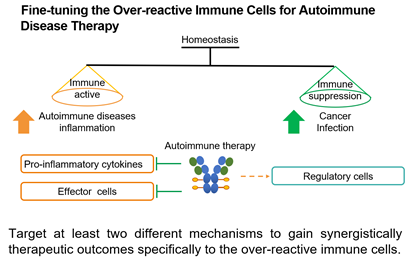
3
Research Ideas for the New Generation of Immunomodulatory ADCs
This article is from Ying’en Biotech, and Yi Yao Guan Lan has obtained authorization for publication from Ying’en Biotech.
Disclaimer: The content team of WuXi AppTec focuses on introducing global biomedical health research progress. This article is for informational exchange purposes only, and the views expressed do not represent the position of WuXi AppTec, nor do they represent WuXi AppTec’s support or opposition to the views expressed. This article is not a treatment recommendation. For treatment guidance, please visit a formal hospital.
