
As of now, the toxicity and prevention measures of ADC in solid tumors are known as follows:
① The indications for ADCs are rapidly expanding, gradually shifting from late-stage to early-stage, and from single therapy to combination strategies.
② The toxicity characteristics of most ADCs are similar to the cytotoxicity of their payloads.
③ Certain ADCs may also exhibit unconventional and potentially life-threatening toxicities, necessitating a better understanding of these events and optimizing diagnostic and management practices.
④ The industry is seeking various pharmacological modification strategies to attempt to improve the tolerability of ADCs, including molecular changes to the antibody portion, linker, and/or cytotoxic payload.
⑤ Exploring different dosing in randomized trials and investigating dose strategies that adapt to responses can optimize the use of ADCs, maximizing their therapeutic value for each indication.
01
Approved ADCs for Solid Tumors: Structure, Major Toxicities, and Interpretation of Causes
So far, the FDA and EMA have approved six ADC drugs for patients with solid tumors.The following figure describes the composition of each ADC (in terms of targeted antigens, monoclonal antibody types, payloads, and linkers), currently approved indications, and the most common toxicities observed for each drug (as shown in Figure 1 and Table 1). The adverse reaction characteristics of each ADC are typically a mix of targeted and off-target effects, with the latter often determining the maximum tolerated dose. Common adverse reactions observed to varying degrees in many ADCs include fatigue, hair loss, blood cell reduction, and gastrointestinal disorders.
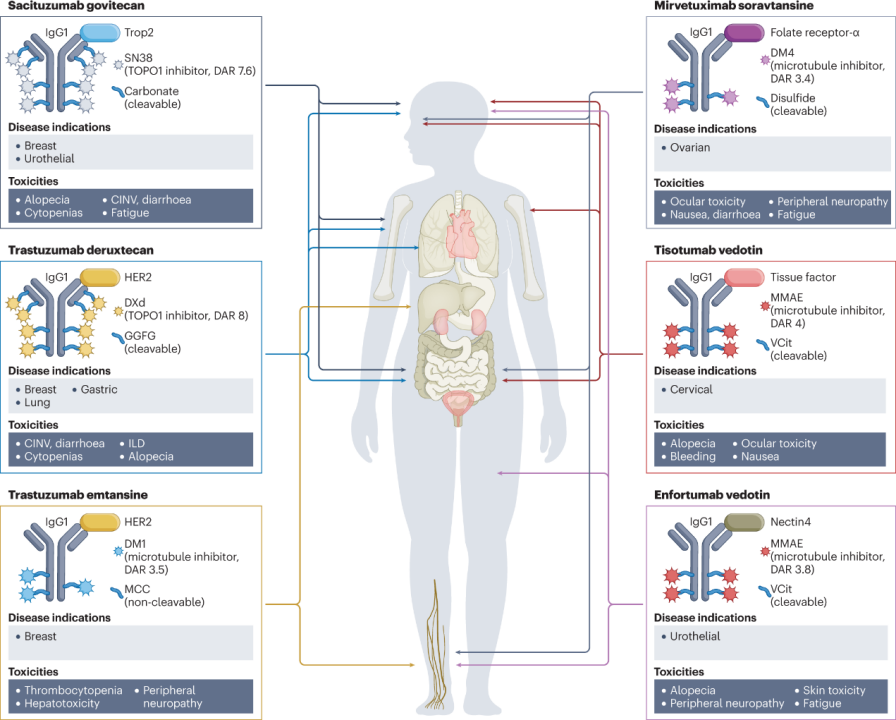
Figure 1: Structures and Major Toxicities of Currently Approved ADCs for Solid Tumors
(Figure Legend: CINV, chemotherapy-induced nausea and vomiting; GGFG, Gly-Gly-Phe-Gly; DAR, drug-antibody ratio; DXd, deruxtecan; ILD, interstitial lung disease; MCC, maleimide methyl cyclohexyl-1-carboxylate; MMAE, monomethyl auristatin E; TOPO1, topoisomerase 1; Trop2, trophoblast cell surface antigen 2; VCit, valine-citrulline.)
Table 1: FDA Approved ADCs Toxicities in Patients with Solid Tumors
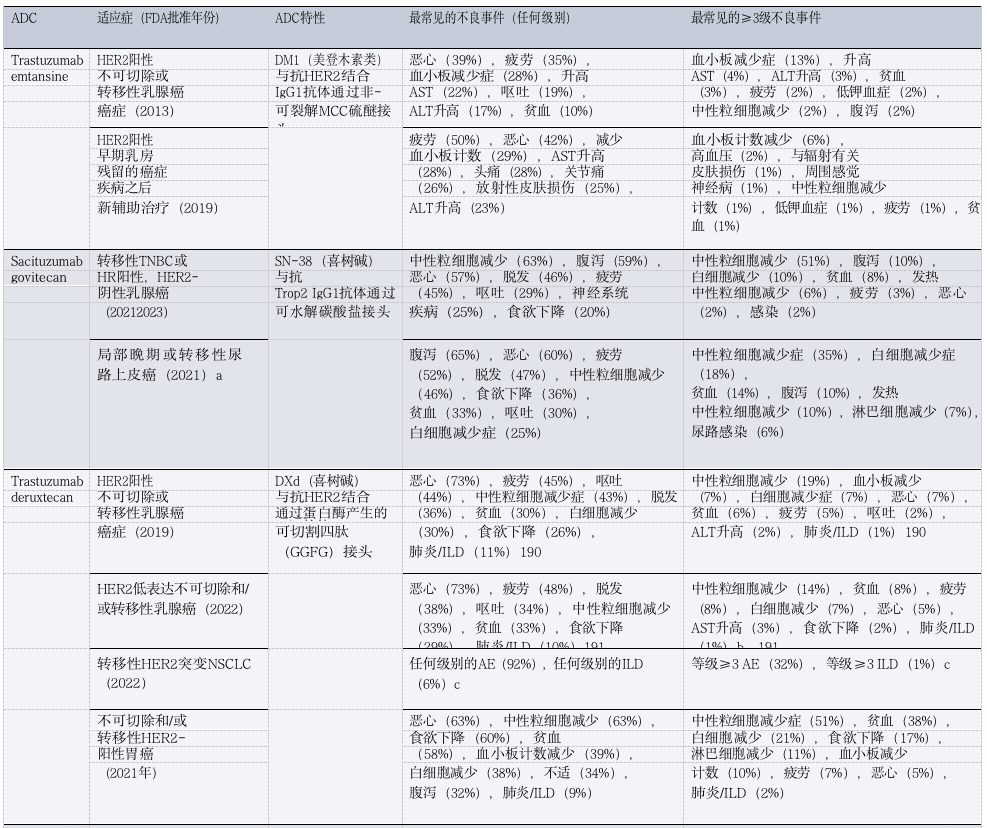
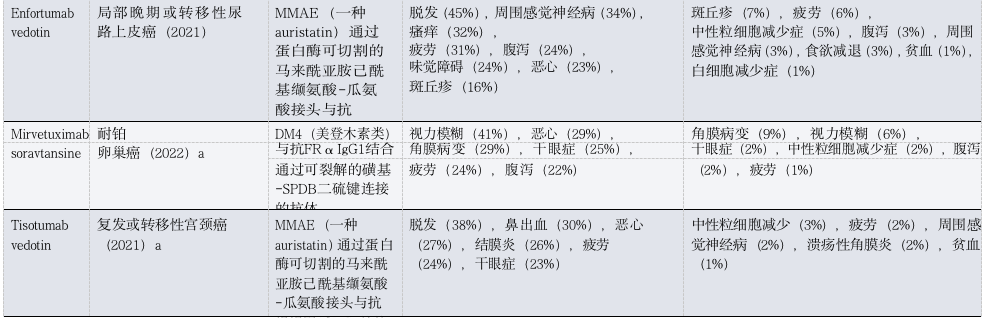
(Table Legend: ADC, antibody-drug conjugate; AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DXd, deruxtecan; FRα, folate receptor-α; GGFG, Gly-Gly-Phe-Gly; HR, hormone receptor; ILD, interstitial lung disease; MCC, maleimide methyl cyclohexyl-1-carboxylate; MMAE, monomethyl auristatin E; NSCLC, non-small cell lung cancer; TNBC, triple-negative breast cancer; Trop2, trophoblast cell surface antigen 2. a Approved only. b Includes three treatment-related deaths. c. Data available only in conference abstracts.)
Due to their large molecular weight, ADCs are usually administered via intravenous injection. In vivo experimental data indicate that this route of administration may be associated with the reduced activity of certain ADCs and severe skin toxicity. Ideally, ADCs should remain intact in circulation after injection and only release their cytotoxic payloads inside or near targeted tumor cells.
Minor changes in the structure of ADCs can often lead to significant changes in the pharmacokinetics and/or pharmacodynamics of the drug (Table 2).
After injection, ADCs typically circulate in the bloodstream as a dynamic mixture of intact ADCs (>90% of the composition), unbound drugs (or drug-linkers), and dissociated antibodies. From circulation, ADCs gradually diffuse into the interstitial space of body tissues, ultimately reaching targeted tumor cells, estimated to be about 0.1% of solid tumors..
Once ADCs reach the tumor microenvironment (TME), they are generally believed to bind to the target antigens expressed on the surface of cancer cells, undergo endocytosis, and release their payloads through chemical or enzymatic cleavage in lysosomes, ultimately leading to necrosis or apoptosis, depending on the mechanism of action of the payload and the concentration achieved at the target site.
More hydrophobic payloads (e.g., monomethyl auristatin E (MMAE) and exatecan derivatives) can diffuse beyond the target cells after dissociating from the antibody within the cell, thereby exerting a “bystander killing” effect on antigen-negative cells, which can enhance the antitumor activity of certain ADCs.
This effect also constitutes a determinant of toxicity: the released payload can also enter adjacent non-malignant cells through passive diffusion or transport protein-mediated uptake, potentially leading to off-target cytotoxicity.
In addition to the traditionally recognized mechanisms of intracellular targeted payload release in tumor cells, certain ADCs can release cytotoxic payloads without antigen involvement and endocytosis. For example, sacituzumab-govitecan has been shown to release its SN-38 payload extracellularly within the TME, a mechanism that may explain the activity and toxicity of this and other ADCs.
Table 2: Pharmacological Determinants of ADC Toxicity
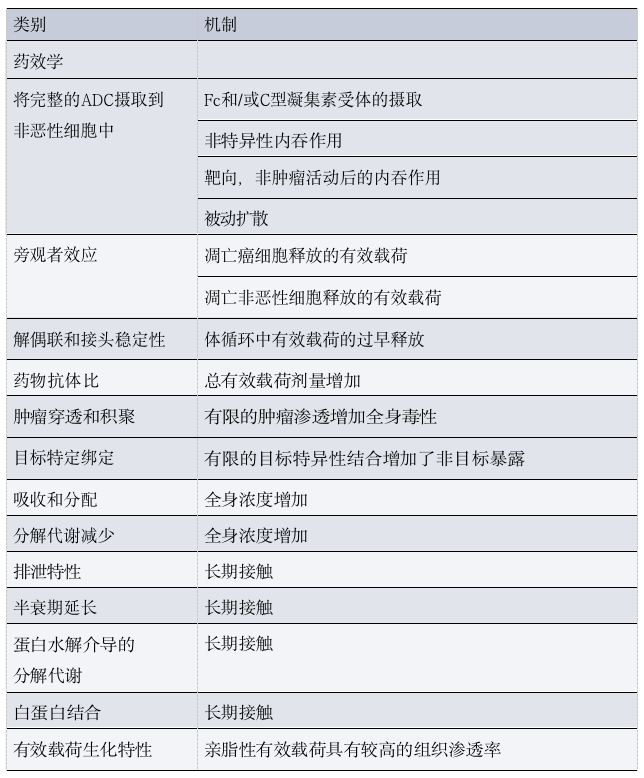
As mentioned above, most components of ADCs do not reach tumor cells and gradually degrade through a combination of specific and non-specific mechanisms before elimination, including target-mediated clearance, Fcγ receptor (FcγR)-mediated uptake, and/or phagocytosis by macrophages located in various tissues.
02
Discussing ADC Toxicity (Starting from Structure)
ADCs have a modular structure, and minor modifications to any of their key components can lead to significant changes in clinical characteristics (Figure 2). This section will analyze the relative contributions of each ADC component and the role of patient characteristics in the types and severity of observed toxicities.
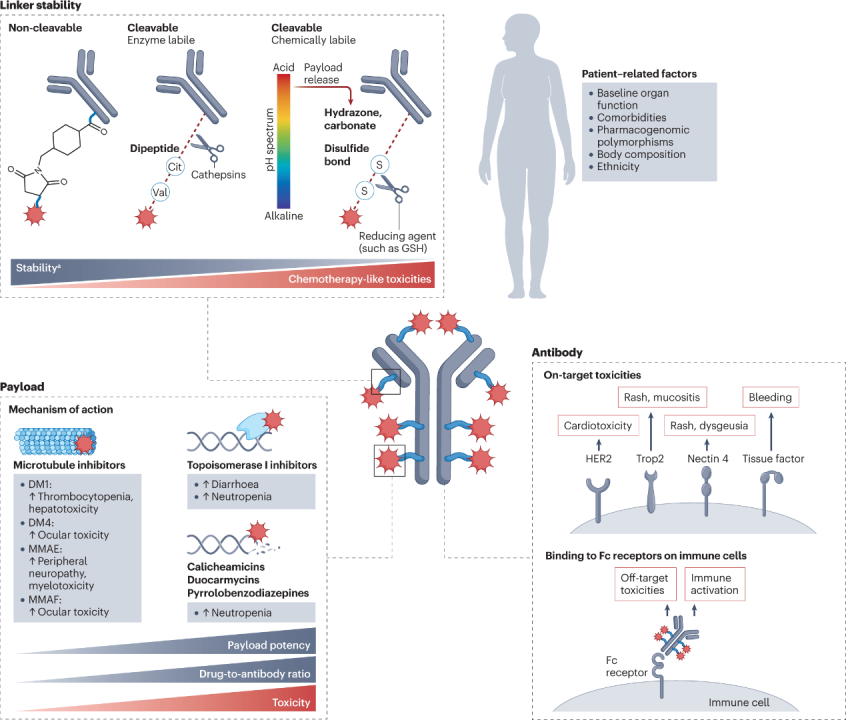
2.1 Payloads
According to the fundamental principles behind ADC development, the targeted antigen is expected to determine the toxicity characteristics of the drug. However, clinical experience indicates that most adverse events associated with ADCs are similar in spectrum, incidence, and severity to the payload backbone, and different ADCs sharing the same payload often exhibit similar toxicity characteristics, regardless of differences in target antigens.
These toxicities can be broadly categorized into off-target, non-tumor effects unrelated to the targeted antigen, and targeted, non-tumor effects resulting from antibody binding to homologous antigens located in non-malignant tissues.
Off-target, non-tumor toxicity dominates the toxicity characteristics of most ADCs, typically leading to adverse reaction profiles similar to that of the payload.
The key mechanisms of off-target, non-tumor toxicity for ADCs are believed to be at least partially related to the premature decoupling of the payload in systemic circulation, leading to the diffusion of free cytotoxic payloads into non-tumor compartments. This payload is typically a lipophilic molecule that can permeate cell membranes and enter non-target non-malignant cells.
2.2 Linkers
As mentioned above, the main mechanisms leading to off-target toxicity in patients receiving ADCs may relate to the timing and localization of payload release from the conjugate. These characteristics largely depend on the stability and pharmacological structure of the linker, and therefore can have a significant impact on the toxicity characteristics of ADCs.
The ideal linker should be stable enough to deliver the payload to the intended site but also unstable enough to release an effective amount of the payload within or near the tumor.
In general, less stable linkers lead to the earlier release of free payloads into circulation, resulting in higher peak concentrations of cytotoxic agents and increased typical chemotherapy-related toxicities (such as cytopenias, hair loss, and/or gastrointestinal toxicity). More stable linkers can lead to prolonged circulation of intact ADCs and delayed release of the payload. This aspect may explain the unique toxicity characteristics of certain highly stable ADCs, some of which have been found to have limited chemotherapy-related toxicities but unexpectedly high rates of ocular toxicity. These findings suggest that a balance should be maintained when determining ADC stability, as excessive release and retention of ADC payloads may lead to unintended toxicities.
In addition to linker stability, the specific chemicals used to conjugate the payload to the antibody and the drug-antibody ratio (DAR) may also impact the toxicity characteristics of ADCs.
2.3 Antibodies
2.4 Patient-Related Factors
In addition to the observed differences in toxicity characteristics across various ADCs, there is also a degree of heterogeneity in the spectrum and grade of adverse reactions occurring in different patients receiving the same ADC. Multiple patient-related factors may influence the pharmacokinetics and pharmacodynamics of these drugs, including baseline organ function, the presence of comorbidities, and polymorphisms of enzymes involved in the metabolism or degradation of ADCs. Finally, ethnicity has been found to affect ADC metabolism: for example, Japanese patients have been found to have an average serum T-DXd concentration 20% higher than patients from other countries, a finding that may explain the higher incidence of ILD observed in that patient population.
03
Adverse Reactions Associated with Combination Therapy Strategies
3.1 ADC Combined with Chemotherapy
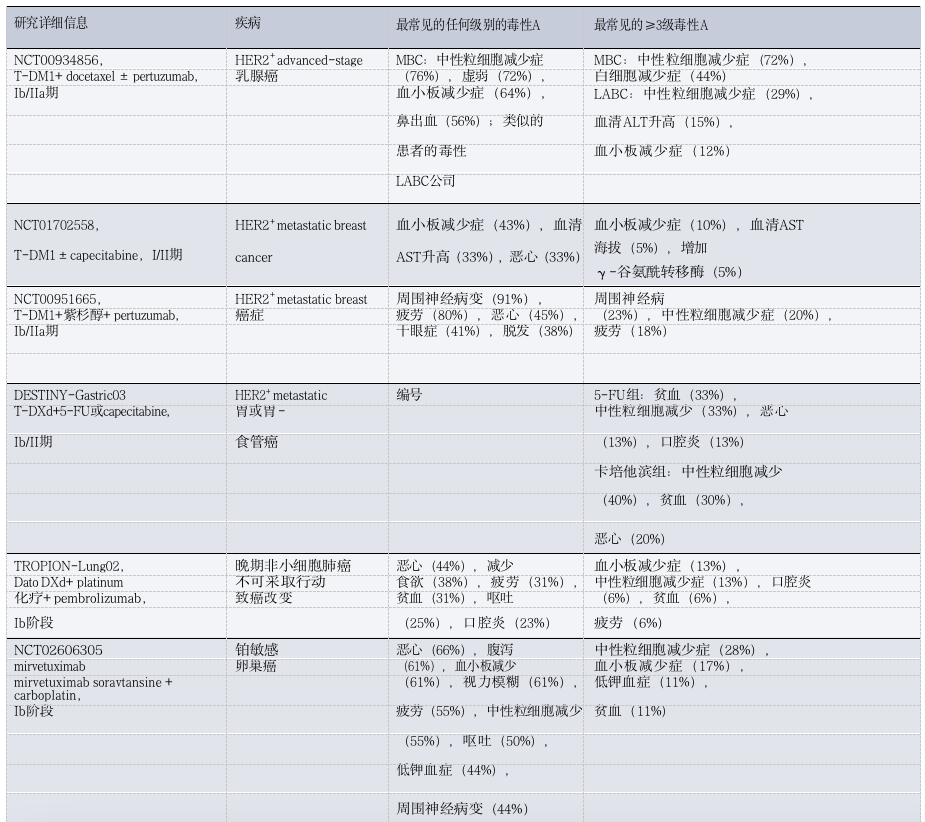
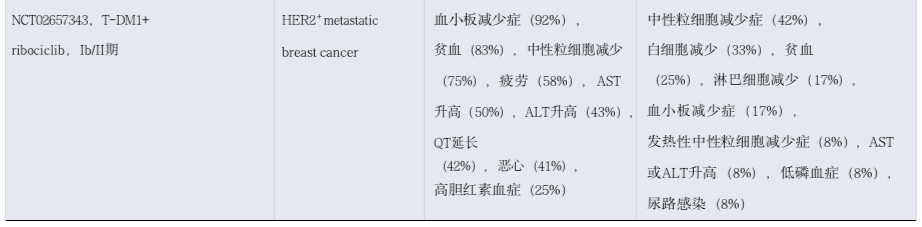
Table 3: Toxicity Observations of ADC Combined with Chemotherapy in Patients with Solid Tumors

3.2 ADC Combined with Endocrine Therapy
3.3 ADC Combined with Immunotherapy
3.4 ADC Combined with Targeted Therapy
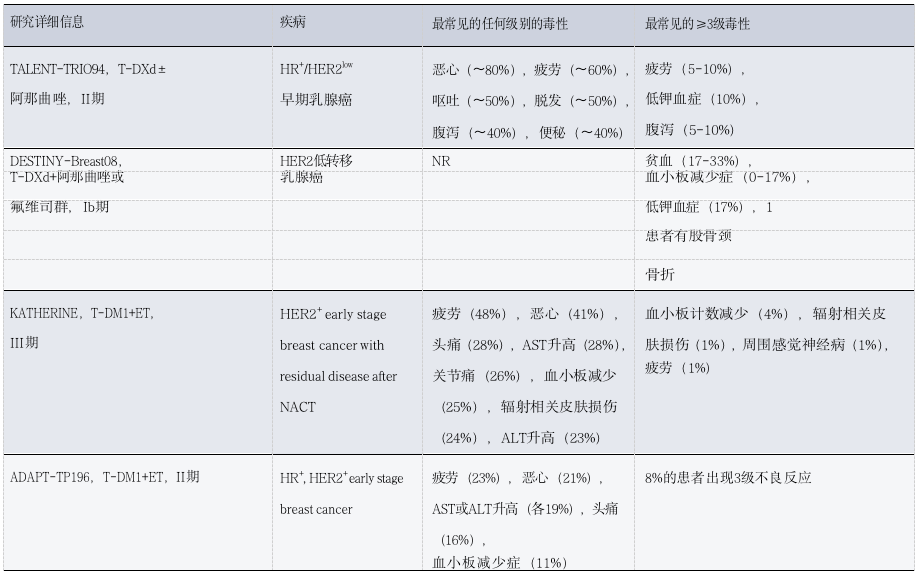
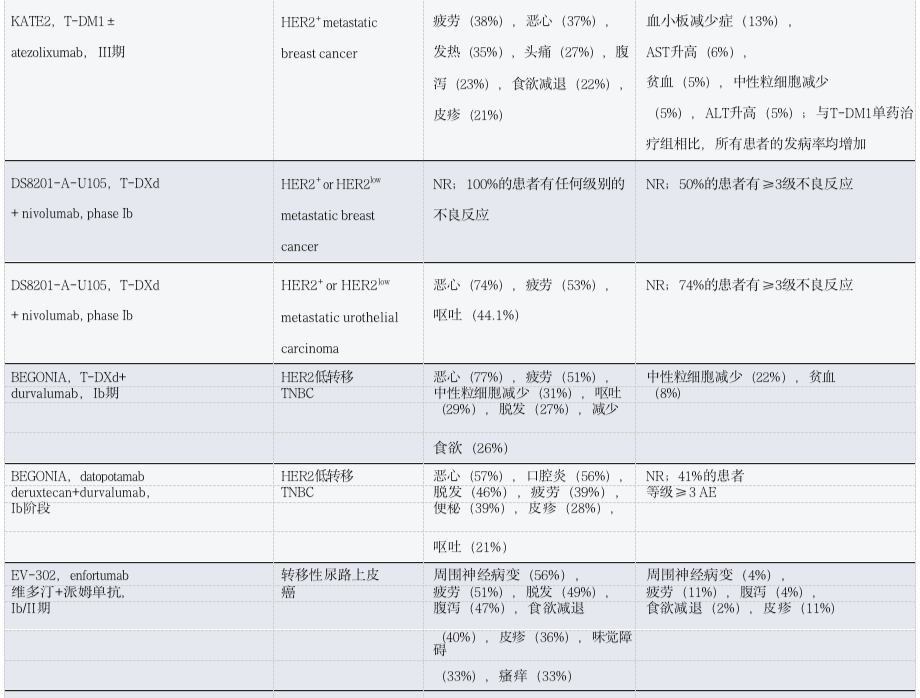
Table 4: Toxicity Observations of ADC Combined with Other Therapies in Patients with Solid Tumors
04
Emerging Strategies to Optimize ADC Safety
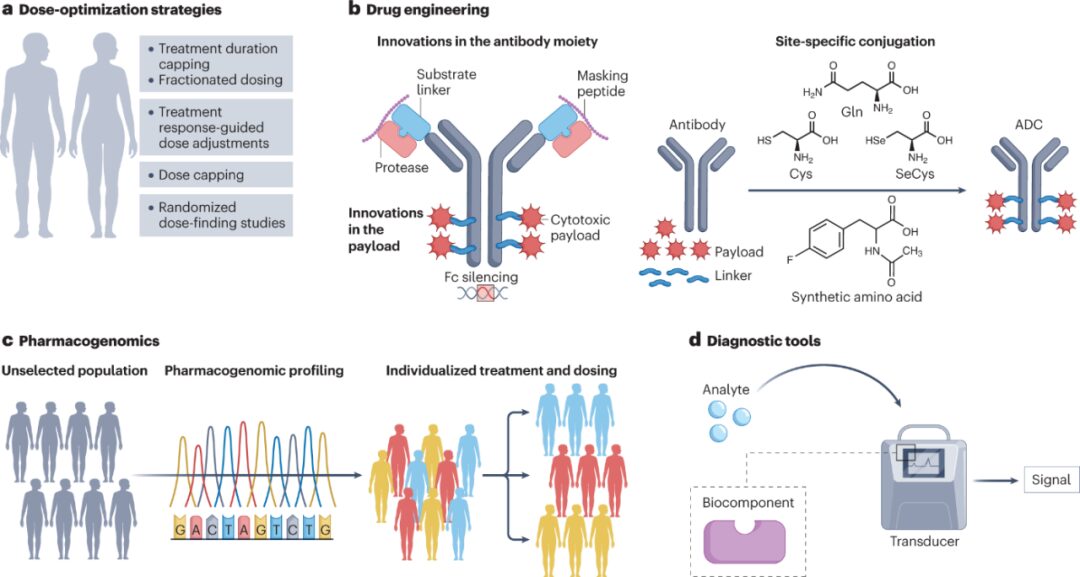
Several strategies have been adopted in clinical practice to prevent or optimize the management of toxicity associated with ADCs (Figure 3).
4.1 Dose Optimization Strategies
Given the dose-dependent nature of many toxicities associated with ADCs, there is considerable interest in optimizing dosing and administration regimens to try to improve the therapeutic index. To this end, five classic dose optimization strategies have been adopted, including weight-based dosing caps, maximum treatment duration caps, dose frequency optimization, response-guided dose adaptation, and randomized dose-finding studies.
4.2 Optimizing ADC Design
4.3 Innovations in Antibody Components
4.4 Innovations in Linker Technologies
4.5 Innovations in Payloads
4.6 Pharmacogenomics
4.7 Diagnostic Tools
Another potential approach to early detection and better management of ADC-induced adverse events involves the use of wearable biosensors (WBS). The rapid development of these technologies may assist in the early diagnosis of ILD in at-risk patients, help identify acute exacerbations of ILD, and support real-time clinical decision-making.
Summary and Outlook
 Main References
Main References
[1] Tarantino P, Ricciuti B, Pradhan SM, Tolaney SM. Optimizing the safety of antibody-drug conjugates for patients with solid tumours. Nat Rev Clin Oncol. 2023 Aug;20(8):558-576. doi: 10.1038/s41571-023-00783-w. Epub 2023 Jun 9. PMID: 37296177.
[2] Jiang, Z. et al. A multiple center, open-label, single-arm, phase II clinical trial of MRG002,
an HER2-targeted antibody-drug conjugate, in patients with HER2-low expressing advanced or metastatic breast cancer. J. Clin. Oncol. 40, 1102–1102 (2022).
[3] Yamaguchi, K. et al. Trastuzumab deruxtecan in anti–-human epidermal growth factor receptor 2 treatment-naive patients with human epidermal growth factor receptor 2-low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II trial. J. Clin. Oncol. 41, 816–825 (2023).
[4] Nguyen, T. D., Bordeau, B. M. & Balthasar, J. P. Mechanisms of ADC toxicity and strategies
to increase ADC tolerability. Cancers 15, 713 (2023).
[5] Hasan, M. M., Laws, M., Jin, P. & Rahman, K. M. Factors influencing the choice of monoclonal antibodies for antibody–drug conjugates. Drug Discov. Today 27, 354–361 (2022).
[6] Nicolò, E. et al. Combining antibody-drug conjugates with immunotherapy in solid tumors: current landscape and future perspectives. Cancer Treat. Rev. 106, 102395(2022).
[7] Schmid, P. et al. Abstract PD11-08: PD11-08 Trastuzumab deruxtecan (T-DXd) + durvalumab (D) as first-line (1L) treatment for unresectable locally advanced/metastatic hormone receptor-negative (HR−), HER2-low breast cancer: updated results from BEGONIA, a phase 1b/2 study. Cancer Res. 83, PD11-08-PD11-08 (2023).
[8] Waks, A. G. et al. Phase Ib study of pembrolizumab in combination with trastuzumab
emtansine for metastatic HER2-positive breast cancer. J. Immunother. Cancer .
