The Concept and Development History of ADC
The treatment of tumors has always undergone a continuous transformation from theory to application, breaking and transforming again. As early as a century ago, scholars proposed the concept of antibody-drug conjugates (ADCs), vividly calling them “magic bullets,” aimed at targeting specific antigens that may exist on the surface of tumor cells. In 1958, researchers first coupled mouse antibodies with methotrexate for the treatment of leukemia. However, due to issues such as antibody preparation and affinity, research stagnated. It wasn’t until the 1970s that the emergence of monoclonal antibodies and humanized antibodies led to the first ADC drug being approved by the FDA in 2000 for the treatment of acute promyelocytic leukemia. However, limited by issues such as linker cleavage and targeting, the drug was unstable in the body, releasing prematurely before reaching the tumor tissue, leading to severe toxicity and a temporary withdrawal from the market. After years of continuous optimization, the new ADC drugs Adcetris and Kadcyla were approved in 2011 and 2013 for the treatment of Hodgkin’s lymphoma and HR2-positive breast cancer, respectively, leading a new wave of research and becoming a new hotspot and important trend in drug development in the field of oncology, following PD-1/PD-L1.
Structure and Optimization of ADC
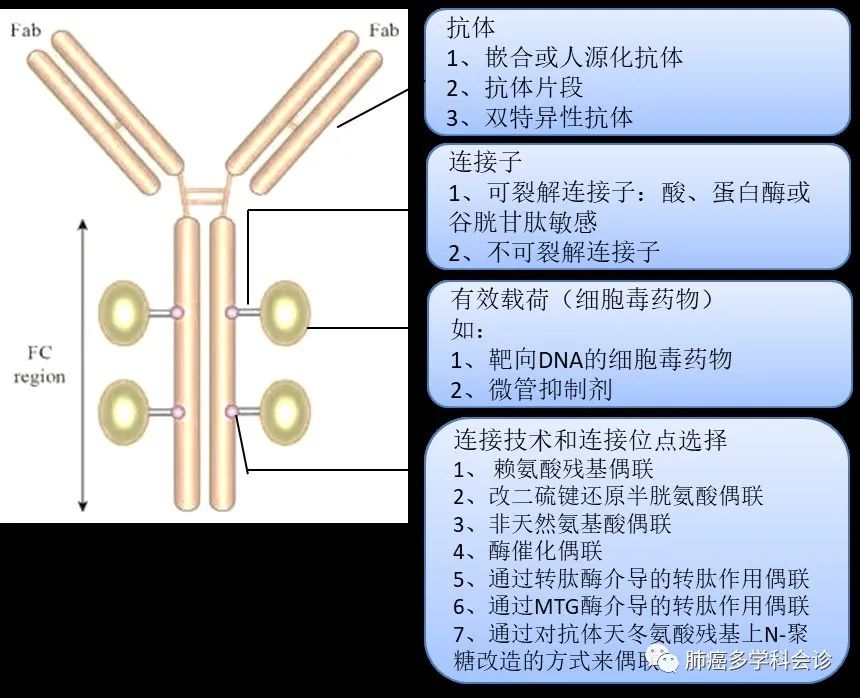
The initial design concept of ADC is very clear: to utilize specific antigens on the surface of tumor cells to recruit monoclonal antibodies, delivering the cytotoxic small molecule drugs they carry directly to the tumor cells or tissues, achieving efficient killing of tumor cells while minimizing toxicity to normal cells and tissues. Therefore, ADC drugs are mainly composed of three parts: small molecule cytotoxic drugs (payload), monoclonal antibodies targeting specific antigens on tumor cell surfaces, and linkers. The advantage of this structure lies in its efficient targeting and ability to kill tumor cells.
Based on the ADC structure, its design needs to consider the following factors: ① Cytotoxic Drugs: Highly effective, non-immunogenic, and capable of binding to the linker after modification; ② Selection of Antibodies: High specificity for the target, high expression in tumor cells while low expression in normal tissues, stability in drug loading, successful internalization by tumor cells, and stable pharmacokinetics; ③ Linker: High stability in circulation to avoid premature cleavage and release, capable of being cleaved by enzymes or released after antibody degradation, ensuring good release inside tumor cells; ④ Linking Sites: Directional coupling and linker payload size, etc.
In terms of targets, the most researched ADCs in China focus on the HER2 target, along with trophoblast cell surface antigen 2 (TROP2), cMET, EGFR, CD20, etc. Different ADC drugs vary based on target selection, the cytotoxic drugs they carry, and linking sites.
Mechanism of Action of ADC Drugs
ADC drugs first enter the systemic circulation through intravenous injection, where antibodies recognize specific antigens, targeting and binding to the surface of tumor cells, and are then internalized into the cells; subsequently, the linker is cleaved by intracellular lysosomal enzymes, releasing the cytotoxic small molecule drugs, while the antibody is degraded; ultimately, the cytotoxic drugs begin to exert efficient killing action, leading to the death of the target cells.
Ideally, if ADC drugs do not rely on internalization, the cytotoxic drugs can be released from the ADC complex into the circulation ahead of time and still exert their effects, thereby stably existing in the tumor microenvironment to exert local anti-tumor effects, also known as the “bystander effect.”
The number of drug molecules loaded onto each antibody molecule is referred to as the drug-antibody ratio (DAR), which is one of the important indicators in evaluating ADC drugs. A high DAR can maximize the delivery of cytotoxic drugs; however, an excessive DAR may affect the overall stability of ADC drugs in circulation and their antigen binding, thereby increasing toxicity and reducing efficacy.
ADC drugs have a wide range of dose-limiting toxicities, including related adverse reactions in the eyes, nerves, and liver, mainly attributed to the off-target effects caused by the premature release of cytotoxic drugs, and may also involve non-specific binding of ADC drugs. Because the cytotoxic molecules in the ADC complex are highly effective, they can exert cytotoxic effects at the picomolar range, requiring that less than 1% of ADCs in circulation are fundamental for maximizing the therapeutic efficacy and safety of tumor-targeted killing. Additionally, the antibody portion of the complex can also cause other adverse events through the process of binding to antigens.
Research Related to ADC Drugs
Given their good targeting and anti-cancer activity, research on ADC drugs is thriving, ushering in a new wave of research and competition. Several HER2-ADC drugs have reached the harvest stage. Currently, two HER2-ADC drugs have been approved in China: the imported trastuzumab emtansine (Kadcyla, T-DM1) and the domestically produced RC48. Additionally, four ADC drugs are in phase III clinical trials, including Enhertu, TAA-013, BAT-8001, and ARX-788. More than ten ADC drugs are undergoing phase I/II clinical trials.
Below we briefly discuss the status of five important ADC drugs:
1. Trastuzumab Emtansine (T-DM1)
T-DM1 is an ADC formed by coupling the HER2-targeting drug trastuzumab and the microtubule inhibitor DM1 (maytansine) through a thioether linker. It is the first drug approved for the treatment of breast cancer. Data (EMILIA) shows that among 991 patients with HER2-positive advanced breast cancer, the median progression-free survival (mPFS) and median overall survival (mOS) in the T-DM1 group were 9.6 months and 30.9 months, significantly longer than the control group (lapatinib + capecitabine, 6.4 months and 25.1 months). T-DM1 treatment significantly extended the OS of patients with brain metastases (26.8 months vs. 12.9 months; P=0.008). Therefore, this ADC drug significantly extends PFS for patients with HER2-positive advanced breast cancer in the second-line setting and successfully translates into OS benefits, achieving OS benefits for patients with brain metastases as well.
A phase II trial, TRAEMOS, has been initiated to evaluate the efficacy and safety of TDM1 combined with osimertinib in advanced EGFR-mutant NSCLC. Let’s stay tuned.
2. Trastuzumab Deruxtecan (T-DXd; DS-8201)
Japan’s Daiichi Sankyo currently has three major ADC therapies, including T-DXd, HER3-DXd, and Dato-DXd, all based on its proprietary DXd system, utilizing a unique cleavable four-peptide linker technology to precisely deliver the effective payload, deruxtecan (Dxd), into tumor cells, achieving efficient killing.
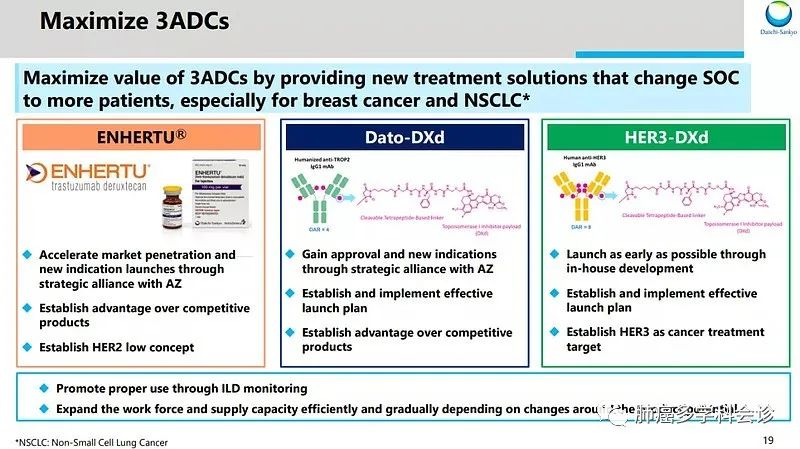
T-DXd is a HER2-targeting ADC inhibitor, formed by coupling trastuzumab with the DXd system, having a higher DAR than T-DM1, indicating greater efficacy. It has recently been approved for HER2-positive breast cancer that is unresectable or metastatic after HER2 treatment, and for locally advanced or metastatic HER2-positive gastric cancer after trastuzumab treatment.
Additionally, the DESTINY-Lung01 trial studied the efficacy of this drug in patients with HER2 overexpression and mutations in NSCLC, showing an ORR of 61.9% and mPFS of 14.0 months in patients with HER2 mutations. Therefore, T-DXd has been granted breakthrough therapy designation by the FDA for advanced NSCLC with HER2 mutations.
However, regarding safety, about 10% of patients treated with T-DXd experienced interstitial lung disease, with approximately 10% of patients discontinuing treatment due to toxicity.
2. HER3-targeting ADC
Patritumab Deruxtecan (HER3-DXd; U3-1402)
Research has confirmed that advanced EGFRm+ NSCLC exhibits upregulation of HER3 expression after TKI resistance, and this patient population has limited treatment options after subsequent progression on platinum-based chemotherapy, necessitating effective treatment strategies.
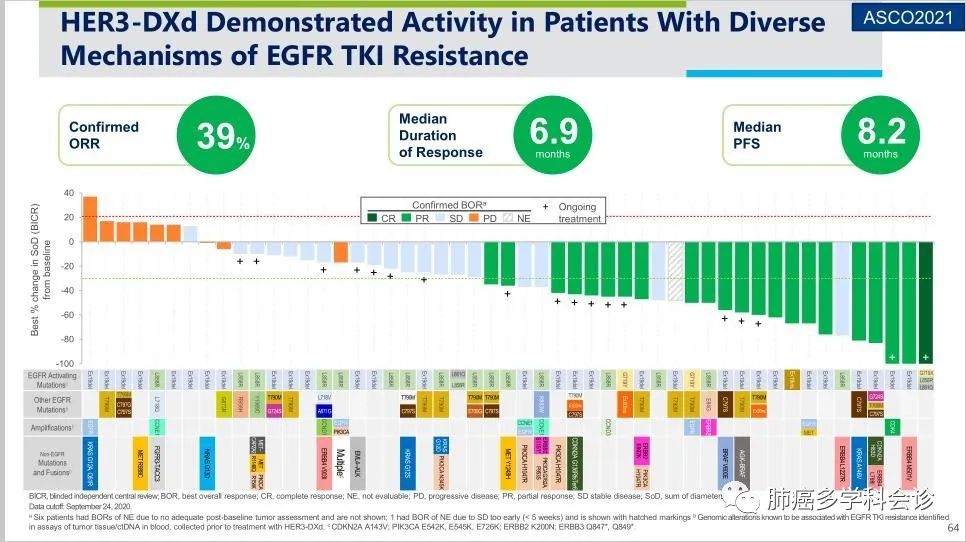
HER3-DXd is a novel ADC targeting HER3, with the antibody component being patritumab monoclonal antibody, which has a higher DAR than trastuzumab, indicating a greater capacity for loading cytotoxic drugs.
The HERTHENA-Lung01 study (Phase I/II) evaluated its efficacy and safety in patients with HER3 mutations after multiple lines of treatment for EGFR 19 deletion/L858R mutated NSCLC. Results showed an ORR of 39%, DCR of 72%, and mPFS of 8.2 months in 57 advanced EGFRm+ NSCLC patients treated with osimertinib. Notably, HER3 overexpression/mutation was not a mandatory inclusion criterion. Regarding safety events, the incidence of ILD (any grade) was 7%.
3. TROP2-targeting ADC
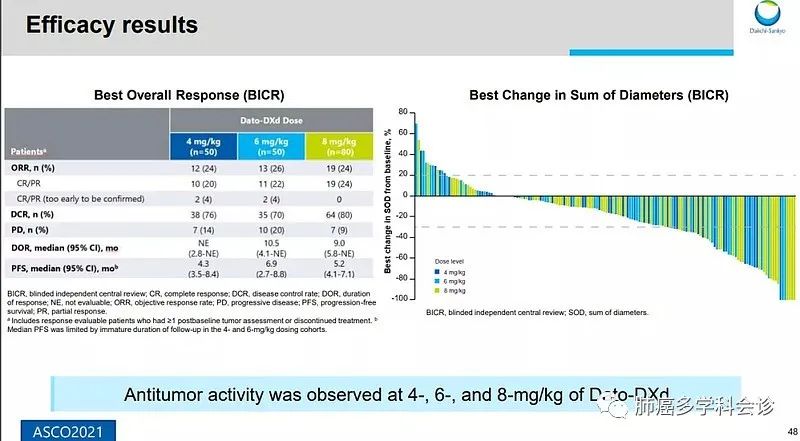
1. Datopotamab-Deruxtecan (Dato-DXd; DS-1062a)
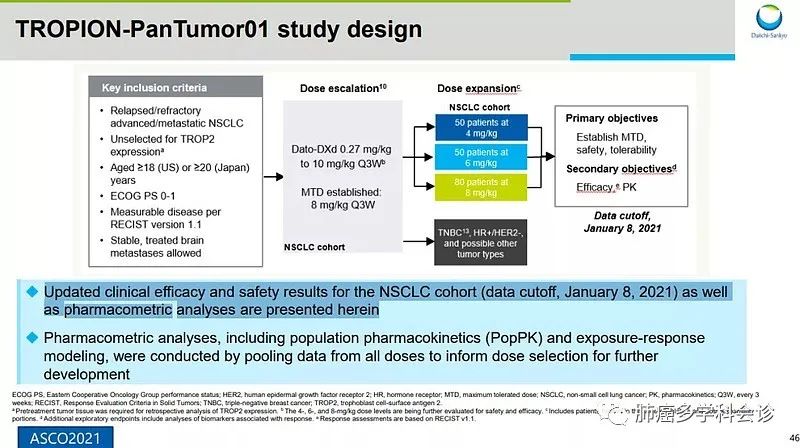
Dato-DXd is an ADC targeting TROP2, utilizing the same DXd system as the above two ADCs, with the antibody component being a humanized anti-TROP2 monoclonal antibody. TROP2, also known as tumor-associated calcium signal transducer 2 (TACSTD2), is expressed in up to 64% of adenocarcinomas and 75% of squamous cell carcinomas, with no effective treatment options available, leading to poor prognosis in these patients.
Data from the TROPION-PanTumor01 study preliminarily showed an ORR of 26% in the 6 mg dose group among NSCLC patients, with a PFS of 6.9 months, and the overall safety profile was manageable, with a rate of treatment-related adverse events (TEAEs) of 16% for grade 3 or higher, including vomiting, anemia, diarrhea, and mucositis.
2. Sacituzumab Govitecan (IMMU-132, SG)
IMMU-132 is an ADC formed by the combination of TROP2 monoclonal antibody and the active metabolite of irinotecan, SN-38. Data (IMMU-132-01 study) showed that its ORR among refractory NSCLC patients was 16.7%, mPFS was 4.4 months, mDOR was 6 months, and mOS was 7.3 months. Another single-arm multicenter study showed that NSCLC patients receiving this drug at 8 or 10 mg/kg had an ORR of 19%, mDOR of 6 months, mOS of 9.5 months, and mPFS of 5.2 months.
References
1. Lambert JM. Antibody-Drug Conjugates (ADCs): Magic Bullets at Last!. Mol Pharm. 2015;12(6):1701–1702. doi:10.1021/acs.molpharmaceut.5b00302
2. Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015 Jan;26(1):113-119.
3. MA11.03 – Trastuzumab Deruxtecan in HER2-Mutated Metastatic Non-Small Cell Lung Cancer (NSCLC): Interim Results of DESTINY-Lung01. Presented at: WCLC 2020 Virtual; January 28-31, 2021.
4. Jänne PA, Baik C, Su WC, et al. Efficacy and safety of U3-1402 (HER3-DXd) in EGFR inhibitor–resistant, EGFR-mutated non–small cell lung cancer. Cancer Discov. Published online September 21, 2021. doi:10.1158/2159-8290.CD-21-0715
5. Heist RS, Guarino MJ,et al.Therapy of Advanced Non-Small-Cell Lung Cancer With an SN-38-Anti-Trop-2 Drug Conjugate, Sacituzumab Govitecan.J Clin Oncol. 2017 May 26:JCO2016721894. doi: 10.1200/JCO.2016.72.1894. [Epub ahead of print]




