-
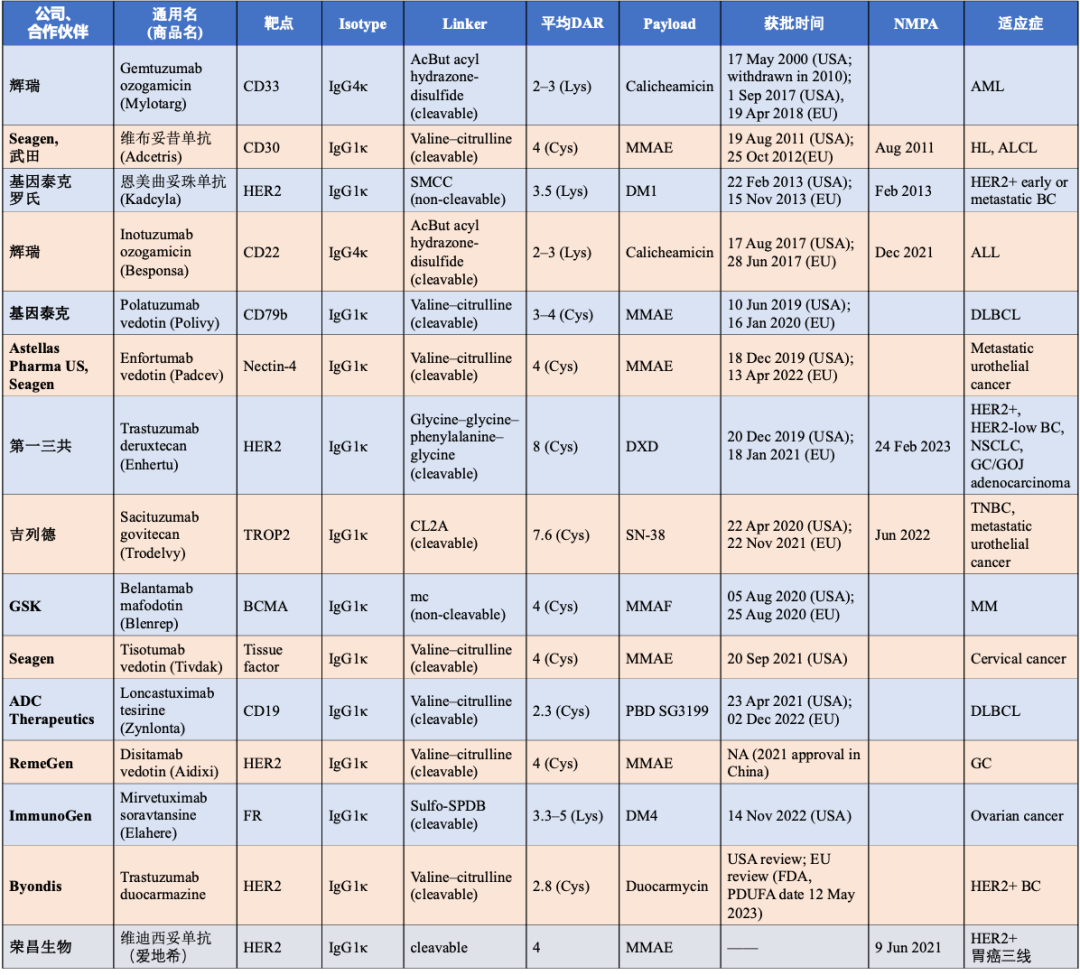
Mylotarg® (gemtuzumab ozogamicin, Gemtuzumab)

-
Adcetris® (brentuximab vedotin, Brentuximab, 安适利®)
-
Kadcyla® (ado-trastuzumab emtansine, Herceptin, 赫赛莱®)
-
Besponsa® (inotuzumab ozogamicin, Inotuzumab, 贝博萨®)
-
Lumoxiti® (Moxetumomab pasudotox, Moxetumomab)
-
Polivy® (polatuzumab vedotin, Polatuzumab, 优罗华®)
-
Enhertu® (fam-trastuzumab deruxtecan-nxki, Enhertu, 优赫得®)

-
Padcev® (enfortumab vedotin, Enfortumab)

-
Trodelvy® (sacituzumab govitecan, Sacituzumab, 拓达维®)
-
Blenrep® (belantamab mafodotin, Belantamab, 玛贝妥单抗)

-
Akalux® (Cetuximab Sarotalocan Sodium, Cetuximab)

-
Zynlonta® (Loncastuximab tesirine, Loncastuximab)

-
Disitamab Vedotin (爱地希®)

-
Tivdak® (tisotumab vedotin-tftv, Tisotumab)
-
Elahere (mirvetuximab soravtansine-gynx, Mirvetuximab)