The human vascular system is a network composed of various blood vessels, including arteries, capillaries, and veins. These vascular structures exhibit significant diversity in shape and structure. For instance, within the vascular system, blood vessels can display different structures in healthy or pathological states, such as stenosis, branching, tortuosity, and aneurysms. The complex structures of these vessels are associated with unique hemodynamics and affect parameters such as flow rate, flow resistance, pressure, and shear stress. These altered flow behaviors can directly impact local biomechanics and cell morphology, leading to significant and unique endothelial cell phenotypes, including activation, coagulation, and innate immune responses.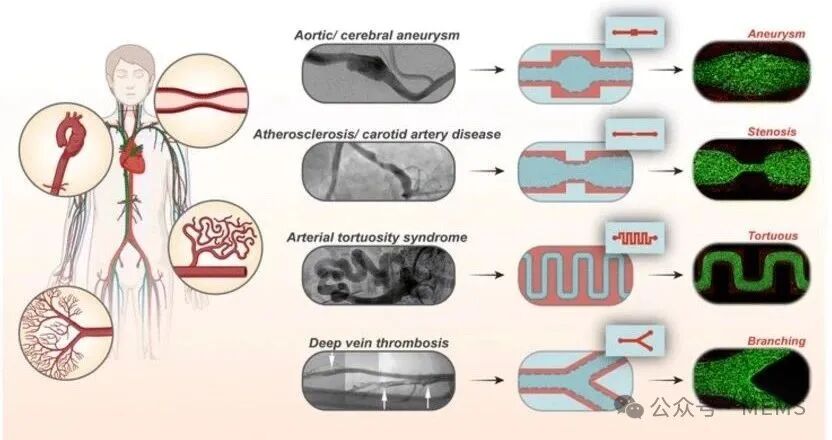
To understand the structure-dependent endothelial pathophysiology, existing vascular model systems are either underdeveloped or difficult to evaluate fundamental biological principles concretely. As an alternative, the recently emerging organ-on-a-chip (OOC) technology can reproduce the physiological environment of human organ systems in a physiologically relevant manner.
Common organ chips consist of microfluidic channels made of polydimethylsiloxane (PDMS) with rectangular cross-sections, and there are also methods for designing circular vascular chips. For example, gravity-driven lumen morphology generation (GLP) is a biomanufacturing technique that has been used for vascular organ chips. GLP is a biomanufacturing strategy that utilizes the interplay of surface tension, gravity, and fluid pressure between two fluids with significantly different viscosities to form cylindrical lumens within microfluidic channels. However, it has not yet been applied to generate more complex vascular structures. A major limitation of existing collagen-embedded vascular chips is the lack of spatial relevance, making it impossible to study various blood vessels with complex structures. Currently, there are microfluidic devices with branches, stenosis, and aneurysms, but there is no unified and straightforward method to rapidly create structurally complex microvessels, including the integration of in vivo endothelial cell culture and blood flow.

According to Maims Consulting, to better capture the complex structures of real human blood vessels, researchers from the Department of Biomedical Engineering at Texas A&M University have developed a customizable vascular chip that enhances the precision of vascular disease research and drug discovery platforms.
The vascular chip is a microfluidic device that simulates human blood vessels on a microscopic scale. Vascular chips can be tailored for specific patients, providing a non-animal method for drug testing and blood flow studies. Jennifer Lee, a master’s student in biomedical engineering at Texas A&M University, joined Dr. Abhishek Jain’s lab to design an advanced vascular chip that can replicate the real changes in vascular structures.
“Some blood vessels have branches, some aneurysms may suddenly expand, and some narrowed vessels can restrict blood flow. All these different types of vessels lead to significant changes in flow patterns, and the inside of the vessels is affected by the shear stress caused by these flow patterns,” Lee said. “This is the model we want to build.”
Researchers replicate different shapes of blood vessels on the vascular chip.
The research findings have been published in the journal Lab on a Chip under the title “Vascular architecture-on-chip: engineering complex blood vessels for reproducing physiological and heterogeneous hemodynamics and endothelial function.”
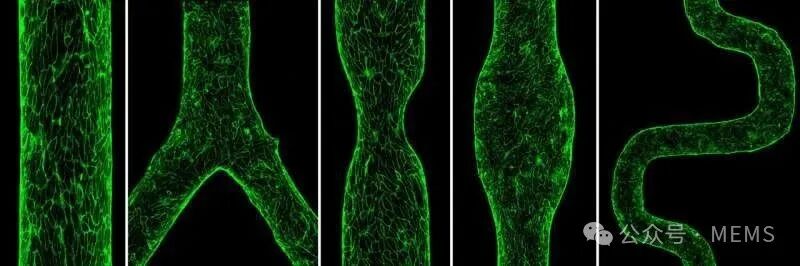
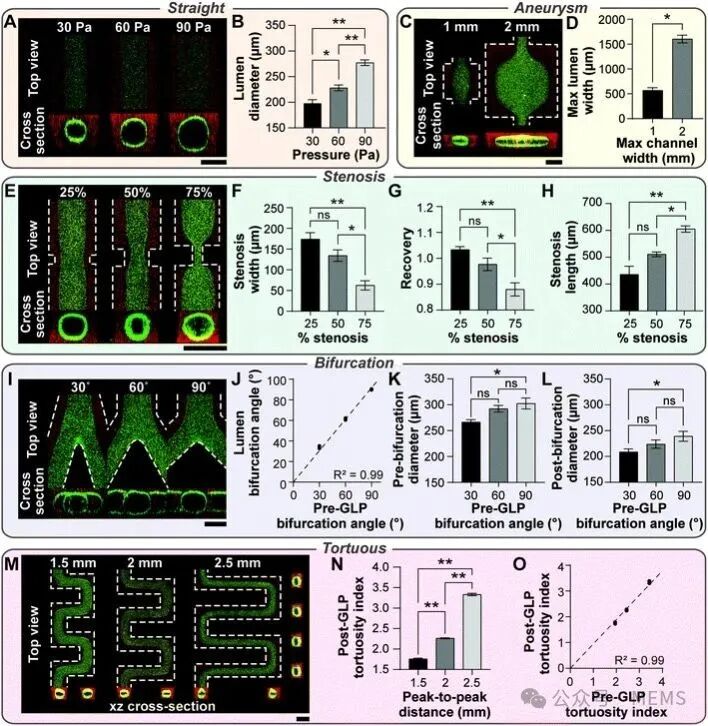
In this study, the researchers used GLP as a unified foundational method to achieve spatial complexity in vascular chips. By inducing viscous fingering through local pressure changes experienced by the two different fluids, the lumen patterns are controlled by the shape and size of the pre-GLP microfluidic channels, resulting in complex lumens of varying shapes and sizes. Using this unified approach, the researchers manufactured vascular chips with different cylindrical diameters, subsequently creating vascular chips with features of stenosis, aneurysms, branching, and tortuosity, and the size and complexity of these vascular chips can be easily controlled and adjusted.
Furthermore, the researchers demonstrated that these vascular chips can be functionalized and cultured with human endothelial cells, allowing them to serve as living pathological vascular substitutes, integrating blood flow and unique hemodynamic patterns.
These easy-to-design yet complex vascular chips reveal the hemodynamic-mediated endothelial functional heterogeneity. For example, endothelial cells exhibit dysfunctional morphological characteristics and abnormal cell arrangements based on hemodynamics. These vascular chips with complex structures can serve as microphysical systems for preclinical vascular disease discovery, localized drug delivery innovations, and mechanisms of cell-cell and cell-drug interactions.
“We are now able to understand vascular diseases in unprecedented ways,” Jain said. “Not only can we make these structures complex, but we can also incorporate actual cells and tissue materials to bring them to life. These are the sites of pathogenesis for vascular diseases, so understanding them is crucial.”
As an undergraduate honors student seeking research experience, Lee entered Jain’s lab. Lee said she knew little about organ-on-a-chip platforms but was intrigued by their potential to reshape future medicine. After entering graduate school, Lee developed a strong interest in vascular chips and joined the Master of Science Fast Track program to continue her newfound research passion.

Vascular chip
While this vascular chip iteration has improved physiological relevance, Jain and Lee hope to expand their research by incorporating various cell types. Lee’s research currently only uses endothelial cells, which make up the inner lining of blood vessels, and they hope to add other cells to observe their interactions and the effects of blood flow.
Construction and experimental study of complex vascular chips
Jain said, “We are continuously making progress, creating what is referred to as the fourth dimension of organ chips, where we not only focus on cells and flow but also on their interactions in more complex structural states, which is a new direction in research in this field.”
In addition to research experience, Lee has gained many soft skills and the ability to apply concepts learned in the classroom to real-world experiences.
She said, “This is a great environment, not only to interact with peers but also to engage with graduate and postdoctoral researchers. We can learn teamwork, communication, work ethic, and try different things. I think this is a valuable experience for students.”
In summary, by adjusting and controlling the GLP method, researchers have designed spatially intricate three-dimensional vascular models. The study shows that the shape and size of patterned lumens can be adjusted through the structure of their external microfluidic channels. Although some representative structures and their variations are currently displayed, this method can be extended to various pathological structural models of blood vessels observed in the human circulation.
In the future, this method could be used to dissect spatially heterogeneous cellular signaling. This includes incorporating surrounding stromal cells into the ECM to create more physiologically relevant in vitro vascular models, which could enhance our understanding of atherosclerosis. Additionally, there is potential to incorporate patient-specific vascular structures and cellular systems, allowing for more personalized drug testing and development.
Paper link:
https://doi.org/10.1039/D4LC00968A
Further reading:
“Biosensor Technology and Market for Instant Diagnostic Applications – 2022 Edition”
“Analysis of Apple’s Patents and Industry Layout in Non-invasive Blood Glucose Monitoring”
“Patent Landscape Analysis of Blood Glucose Monitoring Based on Raman Spectroscopy – 2024 Edition”
“Analysis of Abbott’s Freestyle Libre Continuous Glucose Monitoring Sensor Products”
“Diabetes Management Technology and Market – 2025 Edition”
