Is there a 60W rule for doctors? What common traits do successful doctors share? When did the gap between you and your colleagues start to widen?
Follow “Research Career Insights” for daily tips on survival skills in the medical profession!
Follow now to receive limited-time free resources onmedical research pathways!
 In an article published in the journal Nature Communications, a research team from South Korea explored the construction of islet-specific microenvironments through bioprinting technology to promote the maturation of stem cell-derived islets. Islets are dense clusters of various hormone-producing cell types responsible for blood glucose regulation. However, stem cell-derived islets generated in vitro often lack a three-dimensional extracellular microenvironment and surrounding vascular structures, leading to immature functionality and very low glucose fluctuation detection and insulin release capabilities. The research team successfully constructed an in vivo-like islet microenvironment by optimizing the combination of pancreas-specific extracellular matrix and basement membrane proteins, using bioprinting technology to recreate the spatial patterns surrounding the islets. This bioprinted islet-specific microenvironment facilitated coordinated interactions between the islets and blood vessels, supporting structural and functional characteristics similar to natural islets. This strategy not only improved the functionality of stem cell-derived islets but also provided significant potential for research on islet development, maturation, and diabetes disease modeling, with important implications for future clinical applications.
In an article published in the journal Nature Communications, a research team from South Korea explored the construction of islet-specific microenvironments through bioprinting technology to promote the maturation of stem cell-derived islets. Islets are dense clusters of various hormone-producing cell types responsible for blood glucose regulation. However, stem cell-derived islets generated in vitro often lack a three-dimensional extracellular microenvironment and surrounding vascular structures, leading to immature functionality and very low glucose fluctuation detection and insulin release capabilities. The research team successfully constructed an in vivo-like islet microenvironment by optimizing the combination of pancreas-specific extracellular matrix and basement membrane proteins, using bioprinting technology to recreate the spatial patterns surrounding the islets. This bioprinted islet-specific microenvironment facilitated coordinated interactions between the islets and blood vessels, supporting structural and functional characteristics similar to natural islets. This strategy not only improved the functionality of stem cell-derived islets but also provided significant potential for research on islet development, maturation, and diabetes disease modeling, with important implications for future clinical applications.
 Research BackgroundThe pancreatic islet is a micro-organ composed of various endocrine cells, primarily including insulin-producing β cells, glucagon-producing α cells, and somatostatin-producing δ cells. The pancreatic islet is surrounded by a complex extracellular matrix (ECM) and a widely distributed vascular network, which play a crucial role in maintaining blood glucose balance. The interaction between cells and the ECM, as well as cell adhesion, is vital for the development and maturation of islets. However, stem cell (SC)-derived islets generated in vitro often lack a three-dimensional ECM environment and surrounding vascular structures, leading to immature functionality and an inability to effectively sense glucose fluctuations and release insulin. Despite some progress with multi-stage differentiation protocols, SC-derived islets remain functionally immature, lacking essential microenvironmental factors present in vivo.To overcome the challenges in islet engineering, recent studies have focused on creating in vivo-like microenvironments to promote further maturation of SC-derived islets. The pancreatic ECM contains various proteins that provide physiological signals and mechanical protection for the islets. Basement membrane (BM) proteins are specialized ECM components surrounding the islets, and although they constitute a small proportion of the pancreatic proteome, they play a critical role in supporting the survival and functionality of islets. Studies have shown that SC-derived islets can achieve a more mature state after in vivo transplantation, indicating that they lack certain key factors present in the in vivo environment. To better simulate the in vivo developmental environment, researchers have attempted to introduce endothelial cells (ECs) at different stages of differentiation to promote the maturation of SC-derived islets. However, these studies have primarily focused on two-dimensional environments and have not adequately reproduced the three-dimensional structure of islets and their surrounding vasculature.
Research BackgroundThe pancreatic islet is a micro-organ composed of various endocrine cells, primarily including insulin-producing β cells, glucagon-producing α cells, and somatostatin-producing δ cells. The pancreatic islet is surrounded by a complex extracellular matrix (ECM) and a widely distributed vascular network, which play a crucial role in maintaining blood glucose balance. The interaction between cells and the ECM, as well as cell adhesion, is vital for the development and maturation of islets. However, stem cell (SC)-derived islets generated in vitro often lack a three-dimensional ECM environment and surrounding vascular structures, leading to immature functionality and an inability to effectively sense glucose fluctuations and release insulin. Despite some progress with multi-stage differentiation protocols, SC-derived islets remain functionally immature, lacking essential microenvironmental factors present in vivo.To overcome the challenges in islet engineering, recent studies have focused on creating in vivo-like microenvironments to promote further maturation of SC-derived islets. The pancreatic ECM contains various proteins that provide physiological signals and mechanical protection for the islets. Basement membrane (BM) proteins are specialized ECM components surrounding the islets, and although they constitute a small proportion of the pancreatic proteome, they play a critical role in supporting the survival and functionality of islets. Studies have shown that SC-derived islets can achieve a more mature state after in vivo transplantation, indicating that they lack certain key factors present in the in vivo environment. To better simulate the in vivo developmental environment, researchers have attempted to introduce endothelial cells (ECs) at different stages of differentiation to promote the maturation of SC-derived islets. However, these studies have primarily focused on two-dimensional environments and have not adequately reproduced the three-dimensional structure of islets and their surrounding vasculature. Research FindingsThe research team constructed a specific microenvironment similar to in vivo islets using bioprinting technology to promote the maturation of stem cell-derived islets. By optimizing the combination of pancreas-specific extracellular matrix (ECM) and basement membrane proteins, and utilizing bioprinting technology to reconstruct the spatial structure surrounding the islets, the researchers successfully facilitated coordinated interactions between the islets and blood vessels. This bioprinted islet-specific microenvironment not only improved the functionality of stem cell-derived islets but also provided significant potential for research on islet development, maturation, and diabetes disease modeling, which may play a role in translational applications in the future. The study demonstrated that the bioprinted islet-vascular-ECM network (HICA-V) can effectively simulate the functional coordination of in vivo islets. By using specific geometric guidance during the bioprinting process, the research team successfully reconstructed the spatial arrangement of islets and blood vessels, promoting interactions between islet cells and endothelial cells. This approach significantly enhanced the functional output of the islets, including performance under healthy and inflammatory conditions, approaching the physiological characteristics of natural islets. This research provides new insights into how environmental factors influence islet development, maturation, and diabetes disease modeling, enhancing the potential for translating diabetes treatment into clinical applications.
Research FindingsThe research team constructed a specific microenvironment similar to in vivo islets using bioprinting technology to promote the maturation of stem cell-derived islets. By optimizing the combination of pancreas-specific extracellular matrix (ECM) and basement membrane proteins, and utilizing bioprinting technology to reconstruct the spatial structure surrounding the islets, the researchers successfully facilitated coordinated interactions between the islets and blood vessels. This bioprinted islet-specific microenvironment not only improved the functionality of stem cell-derived islets but also provided significant potential for research on islet development, maturation, and diabetes disease modeling, which may play a role in translational applications in the future. The study demonstrated that the bioprinted islet-vascular-ECM network (HICA-V) can effectively simulate the functional coordination of in vivo islets. By using specific geometric guidance during the bioprinting process, the research team successfully reconstructed the spatial arrangement of islets and blood vessels, promoting interactions between islet cells and endothelial cells. This approach significantly enhanced the functional output of the islets, including performance under healthy and inflammatory conditions, approaching the physiological characteristics of natural islets. This research provides new insights into how environmental factors influence islet development, maturation, and diabetes disease modeling, enhancing the potential for translating diabetes treatment into clinical applications. Clinical SignificanceEnhancing Islet Functional Maturation: By reconstructing the microenvironment of islets, this strategy enhances the structural and functional characteristics of SC-derived islets, improving their insulin secretion and glucose regulation capabilities. This has significant application prospects for diabetes research and treatment. Promoting Islet Vascularization: This technology supports the improvement of structural and functional characteristics of islets by facilitating the synergistic interaction between islets and blood vessels, similar to natural islets. This is a key factor in developing more effective diabetes models and treatment methods. Providing New Strategies for Islet Transplantation: By customizing the islet microenvironment, researchers have demonstrated a new method that can support islet maturation in vitro, opening new avenues for future clinical applications of islet transplantation therapy. Accelerating Diabetes Research: This study provides new tools for research on islet development, maturation, and diabetes pathological models, which may help better understand the pathogenesis of diabetes and develop new treatment methods. By combining bioprinting technology with specific biochemical signals, this research constructs a biological environment that supports islet maturation and functionality, providing new possibilities for basic research and clinical treatment of diabetes.
Clinical SignificanceEnhancing Islet Functional Maturation: By reconstructing the microenvironment of islets, this strategy enhances the structural and functional characteristics of SC-derived islets, improving their insulin secretion and glucose regulation capabilities. This has significant application prospects for diabetes research and treatment. Promoting Islet Vascularization: This technology supports the improvement of structural and functional characteristics of islets by facilitating the synergistic interaction between islets and blood vessels, similar to natural islets. This is a key factor in developing more effective diabetes models and treatment methods. Providing New Strategies for Islet Transplantation: By customizing the islet microenvironment, researchers have demonstrated a new method that can support islet maturation in vitro, opening new avenues for future clinical applications of islet transplantation therapy. Accelerating Diabetes Research: This study provides new tools for research on islet development, maturation, and diabetes pathological models, which may help better understand the pathogenesis of diabetes and develop new treatment methods. By combining bioprinting technology with specific biochemical signals, this research constructs a biological environment that supports islet maturation and functionality, providing new possibilities for basic research and clinical treatment of diabetes. Experimental Strategy1. Microenvironment Reconstruction: ECM Reconstruction: Researchers used decellularized pancreas-derived ECM (pdECM) and basement membrane proteins (such as laminin and type IV collagen) to simulate the pancreatic microenvironment. These components were optimized in a 1:1 ratio to form a bioink called PINE, providing microenvironmental support similar to that surrounding native islets. Protein Analysis: Proteomic analysis was conducted to determine the composition of major ECM proteins in pdECM and compare it with commonly used type I collagen, ensuring that pdECM is closer to the physiological microenvironment of the pancreas.2. Bioprinting: Three-Dimensional Structure Reconstruction: Bioprinting technology, particularly bath bioprinting, was applied to construct tightly integrated three-dimensional networks of islets, blood vessels, and ECM. This method allows for precise replication of pancreas-specific geometric structures. Printing Parameter Optimization: By adjusting printing pressure and nozzle size, the printed islet and vascular structures were ensured to be similar in size and morphology to native islets.3. Functional Validation: Cell Maturity Testing: The maturity of SC-derived islet cells in different bioinks was assessed by detecting the expression of insulin and other islet-specific hormones. Glucose-Stimulated Insulin Secretion (GSIS) Testing: This was used to evaluate the insulin release capability of islet cells under different glucose concentrations. Calcium Ion Signal Analysis: Calcium ion imaging was used to assess the responsiveness of cells to glucose stimulation.4. Drug Testing and Disease Models: Diabetes Model Construction: HICA-V was cultured under high glucose conditions to simulate hyperglycemic-like conditions. Drug Effect Evaluation: The combined effects of antidiabetic drugs (such as metformin and empagliflozin) were tested to observe their impact on islet functionality under high glucose conditions.
Experimental Strategy1. Microenvironment Reconstruction: ECM Reconstruction: Researchers used decellularized pancreas-derived ECM (pdECM) and basement membrane proteins (such as laminin and type IV collagen) to simulate the pancreatic microenvironment. These components were optimized in a 1:1 ratio to form a bioink called PINE, providing microenvironmental support similar to that surrounding native islets. Protein Analysis: Proteomic analysis was conducted to determine the composition of major ECM proteins in pdECM and compare it with commonly used type I collagen, ensuring that pdECM is closer to the physiological microenvironment of the pancreas.2. Bioprinting: Three-Dimensional Structure Reconstruction: Bioprinting technology, particularly bath bioprinting, was applied to construct tightly integrated three-dimensional networks of islets, blood vessels, and ECM. This method allows for precise replication of pancreas-specific geometric structures. Printing Parameter Optimization: By adjusting printing pressure and nozzle size, the printed islet and vascular structures were ensured to be similar in size and morphology to native islets.3. Functional Validation: Cell Maturity Testing: The maturity of SC-derived islet cells in different bioinks was assessed by detecting the expression of insulin and other islet-specific hormones. Glucose-Stimulated Insulin Secretion (GSIS) Testing: This was used to evaluate the insulin release capability of islet cells under different glucose concentrations. Calcium Ion Signal Analysis: Calcium ion imaging was used to assess the responsiveness of cells to glucose stimulation.4. Drug Testing and Disease Models: Diabetes Model Construction: HICA-V was cultured under high glucose conditions to simulate hyperglycemic-like conditions. Drug Effect Evaluation: The combined effects of antidiabetic drugs (such as metformin and empagliflozin) were tested to observe their impact on islet functionality under high glucose conditions. Data InterpretationFigure 1: Proteomic Characteristics of Col and pdECM BioinksFigure 1 shows the proteomic characteristics of Col (collagen) and pdECM (decellularized extracellular matrix) bioinks, revealing their composition and functional properties. A. Optical imaging techniques displayed images of native and decellularized pancreatic tissues. The purpose of the experiment was to demonstrate the impact of the decellularization process on pancreatic tissue. B. A schematic diagram illustrated the development process of PINE bioink, created by BioRender, to explain the preparation process of the bioink. C. The relative abundance of matrix proteins in Col and pdECM was shown by analyzing the percentage of matrix proteins in total proteins. D. The composition of different types of matrix proteins in Col and pdECM was displayed by analyzing the subtypes of matrix proteins. E. A Venn diagram illustrated the total number of proteins identified in Col and pdECM, comparing the protein diversity in the two bioinks. F. GO molecular function analysis showed the functional characteristics of overlapping proteins in Col and pdECM, revealing the similarities in molecular functions of these proteins. G. Cell component analysis displayed the cellular localization of overlapping proteins in Col and pdECM, revealing the distribution of these proteins in cellular structures. H. GO biological process analysis showed the top 7 enriched GOBP terms for proteins identified only in Col, revealing the biological functions of these proteins. I. GO biological process analysis showed the top 7 enriched GOBP terms for proteins identified only in pdECM, revealing the biological functions of these proteins. J. GO biological process analysis illustrated the biological processes of overlapping proteins in Col and pdECM, revealing the roles of these proteins in biological processes. K. The relative abundance of collagen in pdECM was shown by analyzing the matrix abundance of collagen in pdECM. L. The relative abundance of glycoproteins and proteoglycans in pdECM was shown by analyzing their matrix abundance in pdECM. M. Functional classification displayed the functional roles of the top 10 matrix proteins in pdECM, providing foundational data for the development and application of bioinks.
Data InterpretationFigure 1: Proteomic Characteristics of Col and pdECM BioinksFigure 1 shows the proteomic characteristics of Col (collagen) and pdECM (decellularized extracellular matrix) bioinks, revealing their composition and functional properties. A. Optical imaging techniques displayed images of native and decellularized pancreatic tissues. The purpose of the experiment was to demonstrate the impact of the decellularization process on pancreatic tissue. B. A schematic diagram illustrated the development process of PINE bioink, created by BioRender, to explain the preparation process of the bioink. C. The relative abundance of matrix proteins in Col and pdECM was shown by analyzing the percentage of matrix proteins in total proteins. D. The composition of different types of matrix proteins in Col and pdECM was displayed by analyzing the subtypes of matrix proteins. E. A Venn diagram illustrated the total number of proteins identified in Col and pdECM, comparing the protein diversity in the two bioinks. F. GO molecular function analysis showed the functional characteristics of overlapping proteins in Col and pdECM, revealing the similarities in molecular functions of these proteins. G. Cell component analysis displayed the cellular localization of overlapping proteins in Col and pdECM, revealing the distribution of these proteins in cellular structures. H. GO biological process analysis showed the top 7 enriched GOBP terms for proteins identified only in Col, revealing the biological functions of these proteins. I. GO biological process analysis showed the top 7 enriched GOBP terms for proteins identified only in pdECM, revealing the biological functions of these proteins. J. GO biological process analysis illustrated the biological processes of overlapping proteins in Col and pdECM, revealing the roles of these proteins in biological processes. K. The relative abundance of collagen in pdECM was shown by analyzing the matrix abundance of collagen in pdECM. L. The relative abundance of glycoproteins and proteoglycans in pdECM was shown by analyzing their matrix abundance in pdECM. M. Functional classification displayed the functional roles of the top 10 matrix proteins in pdECM, providing foundational data for the development and application of bioinks. Figure 2: Characterization of Stem Cell-Derived Differentiated Islet Cells and Comparative Analysis of Islet MicroenvironmentsFigure 2 presents multiple experiments conducted to assess the differentiation characteristics of stem cell-derived islet cells and their comparison with islet microenvironments. A. The figure illustrates the process of human embryonic stem cells (hESC) differentiating into islet cells and the subsequent workflow. B. Images of cells at stage six were captured under a light microscope, showing morphological characteristics under two-dimensional culture and suspension culture conditions. C. Real-time quantitative PCR analysis was performed to analyze the expression of endodermal and pancreatic genes at different differentiation stages, revealing significant expression of FOXA2 at stage S1, a significant increase in PDX1 expression at stage S4, significant upregulation of NKX6.1 at stage S4, and significant upregulation of INS, GCG, and SST at stage S6. D. Immunostaining detected the expression of PDX1, NKX6.1, C-peptide, CHGA, GCG, and SST in differentiated cells, indicating the expression of these pancreatic markers in differentiated cells. E. Immunostaining compared the expression of LAM, INS, and COL IV in human islets and stem cell-derived islets, showing similarities in the expression of these matrix proteins. Conclusion: Stem cell-derived islet cells exhibit specific gene and protein marker expression patterns during differentiation and show similarities to human islets in the expression of certain matrix proteins, indicating potential structural and functional similarities to natural islets.
Figure 2: Characterization of Stem Cell-Derived Differentiated Islet Cells and Comparative Analysis of Islet MicroenvironmentsFigure 2 presents multiple experiments conducted to assess the differentiation characteristics of stem cell-derived islet cells and their comparison with islet microenvironments. A. The figure illustrates the process of human embryonic stem cells (hESC) differentiating into islet cells and the subsequent workflow. B. Images of cells at stage six were captured under a light microscope, showing morphological characteristics under two-dimensional culture and suspension culture conditions. C. Real-time quantitative PCR analysis was performed to analyze the expression of endodermal and pancreatic genes at different differentiation stages, revealing significant expression of FOXA2 at stage S1, a significant increase in PDX1 expression at stage S4, significant upregulation of NKX6.1 at stage S4, and significant upregulation of INS, GCG, and SST at stage S6. D. Immunostaining detected the expression of PDX1, NKX6.1, C-peptide, CHGA, GCG, and SST in differentiated cells, indicating the expression of these pancreatic markers in differentiated cells. E. Immunostaining compared the expression of LAM, INS, and COL IV in human islets and stem cell-derived islets, showing similarities in the expression of these matrix proteins. Conclusion: Stem cell-derived islet cells exhibit specific gene and protein marker expression patterns during differentiation and show similarities to human islets in the expression of certain matrix proteins, indicating potential structural and functional similarities to natural islets.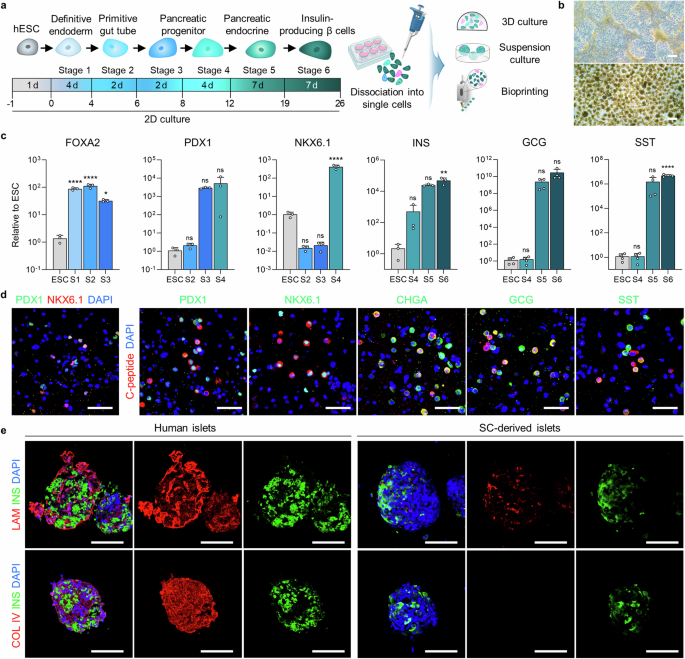 Figure 3: Study on the Impact of Microenvironment on the Maturation of Stem Cell-Derived β CellsFigure 3 investigated the effects of different bioinks on the maturation of stem cell-derived β cells, primarily assessed through immunostaining, gene expression analysis, glucose-stimulated insulin secretion (GSIS) experiments, and calcium signal detection. A. Immunostaining detected the expression of insulin (INS), chromogranin A (CHGA), glucagon (GCG), and somatostatin (SST) in cells encapsulated in different bioinks. The results showed that all groups expressed these hormones, indicating that cells could differentiate and mature in different bioinks. B. Quantitative analysis of the proportion of hormone-positive cells in different groups revealed that the proportion of INS and CHGA positive cells in the PINE bioink group was significantly higher than in the Col and pdECM groups, while the proportion of GCG and SST positive cells showed no significant differences among groups. C. By comparing the relative expression levels of hormone-related genes in each group, it was found that INS gene expression in the PINE bioink group was significantly higher than in the Col and pdECM groups, while CHGA, GCG, and SST gene expression showed no significant differences among groups. D. The glucose-stimulated insulin secretion (GSIS) experiment assessed the responsiveness of cells encapsulated in different bioinks to glucose stimulation. The results showed that under high glucose conditions, insulin secretion from cells in the PINE bioink group was significantly higher than in the Col and pdECM groups. E. Calcium signal detection observed changes in calcium signals in stem cell-derived islet cells encapsulated in different bioinks under high glucose and exendin-4 stimulation. The results showed that the calcium signal intensity in the PINE bioink group was higher than in other groups. F. Quantitative analysis of the peak height of calcium signals in individual stem cell-derived islet cells revealed that the calcium signal peak in the PINE bioink group was significantly higher than in the Col group, while there were no significant differences between the pdECM and Col groups. Conclusion: The PINE bioink significantly promotes the maturation of stem cell-derived β cells, enhancing their responsiveness to glucose stimulation and calcium signal intensity.
Figure 3: Study on the Impact of Microenvironment on the Maturation of Stem Cell-Derived β CellsFigure 3 investigated the effects of different bioinks on the maturation of stem cell-derived β cells, primarily assessed through immunostaining, gene expression analysis, glucose-stimulated insulin secretion (GSIS) experiments, and calcium signal detection. A. Immunostaining detected the expression of insulin (INS), chromogranin A (CHGA), glucagon (GCG), and somatostatin (SST) in cells encapsulated in different bioinks. The results showed that all groups expressed these hormones, indicating that cells could differentiate and mature in different bioinks. B. Quantitative analysis of the proportion of hormone-positive cells in different groups revealed that the proportion of INS and CHGA positive cells in the PINE bioink group was significantly higher than in the Col and pdECM groups, while the proportion of GCG and SST positive cells showed no significant differences among groups. C. By comparing the relative expression levels of hormone-related genes in each group, it was found that INS gene expression in the PINE bioink group was significantly higher than in the Col and pdECM groups, while CHGA, GCG, and SST gene expression showed no significant differences among groups. D. The glucose-stimulated insulin secretion (GSIS) experiment assessed the responsiveness of cells encapsulated in different bioinks to glucose stimulation. The results showed that under high glucose conditions, insulin secretion from cells in the PINE bioink group was significantly higher than in the Col and pdECM groups. E. Calcium signal detection observed changes in calcium signals in stem cell-derived islet cells encapsulated in different bioinks under high glucose and exendin-4 stimulation. The results showed that the calcium signal intensity in the PINE bioink group was higher than in other groups. F. Quantitative analysis of the peak height of calcium signals in individual stem cell-derived islet cells revealed that the calcium signal peak in the PINE bioink group was significantly higher than in the Col group, while there were no significant differences between the pdECM and Col groups. Conclusion: The PINE bioink significantly promotes the maturation of stem cell-derived β cells, enhancing their responsiveness to glucose stimulation and calcium signal intensity.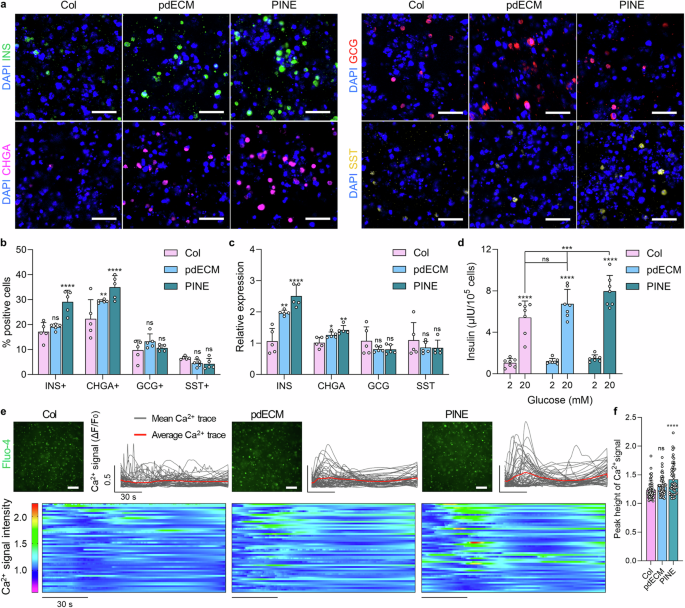 Figure 4: Evaluation of the Rheological Properties and Geometric Control of HICA-VFigure 4 presents the experimental results regarding the rheological properties and geometric control of HICA-V, involving the self-healing properties of the bioink, shear-thinning behavior, and cell viability during the printing process. a. The figure illustrates a schematic of islet-specific cell tissue printing using SC-derived islet cells and endothelial cells, created by BioRender, showcasing the design of the printing process. b-d. The self-healing properties of Col, pdECM, and PINE bioinks were evaluated under low (1%) and high (300%) strain cycles, showing that these bioinks exhibit good self-healing performance. e. The shear-thinning behavior of each bioink was analyzed, revealing that they exhibited shear-thinning characteristics under shear forces. f. The figure shows the positioning and movement of the nozzle during the bath printing process of HICA-V, ensuring precision and controllability in printing. g. Live/dead staining assessed the cell viability of HICAs and blood vessels under different air pressure conditions, indicating that under appropriate air pressure, cell survival rates were high. h. Live/dead staining evaluated the cell viability of HICA-V under optimized printing conditions on day 1 and day 4 (left side), as well as the visualization of the 3D-rendered structure of HICA-V (right side), showing high cell survival rates and structural integrity under optimized conditions. i. Immunostaining of F-actin, CD31, and DAPI at different time points for HICA-V showed the expression of cytoskeletal and vascular markers, validating the functionality and structural integrity of the cells. Conclusion: HICA-V bioink exhibits good rheological properties and geometric control during the printing process, supporting high cell viability and functional expression.
Figure 4: Evaluation of the Rheological Properties and Geometric Control of HICA-VFigure 4 presents the experimental results regarding the rheological properties and geometric control of HICA-V, involving the self-healing properties of the bioink, shear-thinning behavior, and cell viability during the printing process. a. The figure illustrates a schematic of islet-specific cell tissue printing using SC-derived islet cells and endothelial cells, created by BioRender, showcasing the design of the printing process. b-d. The self-healing properties of Col, pdECM, and PINE bioinks were evaluated under low (1%) and high (300%) strain cycles, showing that these bioinks exhibit good self-healing performance. e. The shear-thinning behavior of each bioink was analyzed, revealing that they exhibited shear-thinning characteristics under shear forces. f. The figure shows the positioning and movement of the nozzle during the bath printing process of HICA-V, ensuring precision and controllability in printing. g. Live/dead staining assessed the cell viability of HICAs and blood vessels under different air pressure conditions, indicating that under appropriate air pressure, cell survival rates were high. h. Live/dead staining evaluated the cell viability of HICA-V under optimized printing conditions on day 1 and day 4 (left side), as well as the visualization of the 3D-rendered structure of HICA-V (right side), showing high cell survival rates and structural integrity under optimized conditions. i. Immunostaining of F-actin, CD31, and DAPI at different time points for HICA-V showed the expression of cytoskeletal and vascular markers, validating the functionality and structural integrity of the cells. Conclusion: HICA-V bioink exhibits good rheological properties and geometric control during the printing process, supporting high cell viability and functional expression.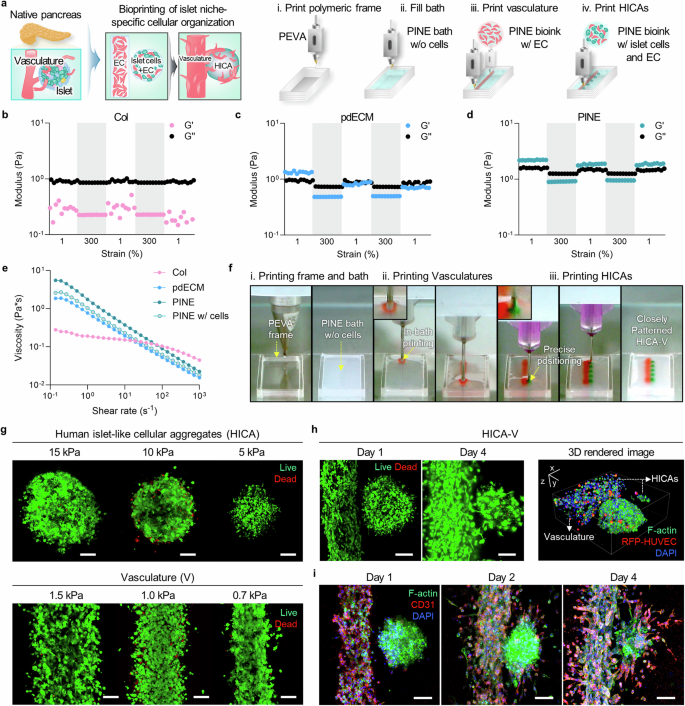 Figure 5: Functional Validation of HICA-V Compared to Random and Suspension GroupsFigure 5 aims to validate the performance of HICA-V in terms of cell functionality and compare it with random and suspension groups. a. The figure illustrates the interactions and connections between β cells and endothelial cells (EC) and the design of the experimental groups. b. Immunostaining of CD31 in HICA-V on day 4 showed the expression of CD31 in HICA-V. This experiment was repeated three times with consistent results. c. Immunostaining of INS and CD31 in each group on day 1 and day 4 showed the expression of INS and CD31 at different time points in each group. This experiment was repeated three times with consistent results. d, e. qPCR analysis compared the gene expression of human islets (H islets), random dispersed group (Random), suspension culture group (Suspension), and HICA-V group. The results showed significant differences in the expression of E-CAD, CX36, VE-CAD, CX43, INS, and FGF genes in the HICA-V group compared to other groups. f. Comparison of glucose-stimulated insulin secretion (GSIS) among groups showed that the HICA-V group had significantly higher insulin secretion under 20 mM glucose stimulation than other groups. g. Comparison of stimulation indices among groups showed no significant differences in stimulation indices between the HICA-V group and other groups. Conclusion: HICA-V outperformed random and suspension groups in terms of cell functionality, particularly in gene expression and glucose-stimulated insulin secretion.
Figure 5: Functional Validation of HICA-V Compared to Random and Suspension GroupsFigure 5 aims to validate the performance of HICA-V in terms of cell functionality and compare it with random and suspension groups. a. The figure illustrates the interactions and connections between β cells and endothelial cells (EC) and the design of the experimental groups. b. Immunostaining of CD31 in HICA-V on day 4 showed the expression of CD31 in HICA-V. This experiment was repeated three times with consistent results. c. Immunostaining of INS and CD31 in each group on day 1 and day 4 showed the expression of INS and CD31 at different time points in each group. This experiment was repeated three times with consistent results. d, e. qPCR analysis compared the gene expression of human islets (H islets), random dispersed group (Random), suspension culture group (Suspension), and HICA-V group. The results showed significant differences in the expression of E-CAD, CX36, VE-CAD, CX43, INS, and FGF genes in the HICA-V group compared to other groups. f. Comparison of glucose-stimulated insulin secretion (GSIS) among groups showed that the HICA-V group had significantly higher insulin secretion under 20 mM glucose stimulation than other groups. g. Comparison of stimulation indices among groups showed no significant differences in stimulation indices between the HICA-V group and other groups. Conclusion: HICA-V outperformed random and suspension groups in terms of cell functionality, particularly in gene expression and glucose-stimulated insulin secretion.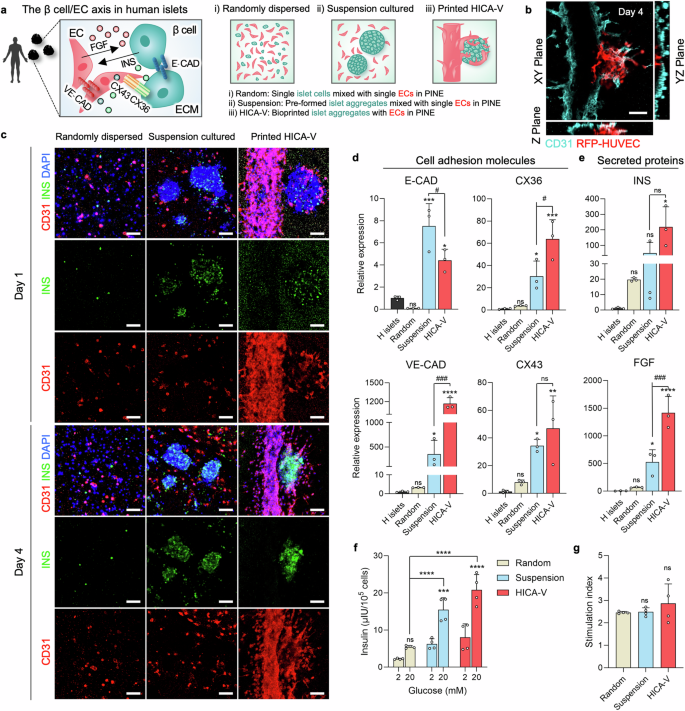 Figure 6: Demonstration of In Vivo Simulation of Pathophysiological Phenomena by Inducing Hyperglycemic-Like Conditions with HICA-VFigure 6 aims to demonstrate the in vivo simulation of pathophysiological phenomena using HICA-V by inducing hyperglycemic-like conditions and treating with antidiabetic drugs. A. The diagram illustrates the experimental design for inducing hyperglycemic-like conditions and treating with antidiabetic drugs using printed HICA-V. B. The time points for high glucose and drug treatment are shown. C. qPCR analysis assessed the expression levels of IL-6 and VCAM-1 in different treatment groups (Met-/Empa-, Met+/Empa-, Met+/Empa+, normal group). The results showed that the IL-6 and VCAM-1 expression in the Met+/Empa+ group was significantly lower compared to the Met-/Empa- group. D. GSIS testing evaluated the insulin secretion in different treatment groups. The results indicated that the Met+/Empa+ group had significantly increased insulin secretion under high glucose stimulation. E. Stimulation index assessments showed that the Met+/Empa+ group had a significantly higher stimulation index than the Met-/Empa- group. F. Immunostaining detected the expression of CD31 and INS in each group on day 8, showing significantly increased CD31 and INS expression in the Met+/Empa+ group. G. Immunofluorescence intensity analysis showed that the fluorescence intensity of CD31 and INS in the Met+/Empa+ group was significantly higher than in the Met-/Empa- group. Conclusion: By inducing hyperglycemic-like conditions and treating with antidiabetic drugs, HICA-V can simulate in vivo pathophysiological phenomena, particularly showing significant changes in insulin secretion and related marker expression.
Figure 6: Demonstration of In Vivo Simulation of Pathophysiological Phenomena by Inducing Hyperglycemic-Like Conditions with HICA-VFigure 6 aims to demonstrate the in vivo simulation of pathophysiological phenomena using HICA-V by inducing hyperglycemic-like conditions and treating with antidiabetic drugs. A. The diagram illustrates the experimental design for inducing hyperglycemic-like conditions and treating with antidiabetic drugs using printed HICA-V. B. The time points for high glucose and drug treatment are shown. C. qPCR analysis assessed the expression levels of IL-6 and VCAM-1 in different treatment groups (Met-/Empa-, Met+/Empa-, Met+/Empa+, normal group). The results showed that the IL-6 and VCAM-1 expression in the Met+/Empa+ group was significantly lower compared to the Met-/Empa- group. D. GSIS testing evaluated the insulin secretion in different treatment groups. The results indicated that the Met+/Empa+ group had significantly increased insulin secretion under high glucose stimulation. E. Stimulation index assessments showed that the Met+/Empa+ group had a significantly higher stimulation index than the Met-/Empa- group. F. Immunostaining detected the expression of CD31 and INS in each group on day 8, showing significantly increased CD31 and INS expression in the Met+/Empa+ group. G. Immunofluorescence intensity analysis showed that the fluorescence intensity of CD31 and INS in the Met+/Empa+ group was significantly higher than in the Met-/Empa- group. Conclusion: By inducing hyperglycemic-like conditions and treating with antidiabetic drugs, HICA-V can simulate in vivo pathophysiological phenomena, particularly showing significant changes in insulin secretion and related marker expression.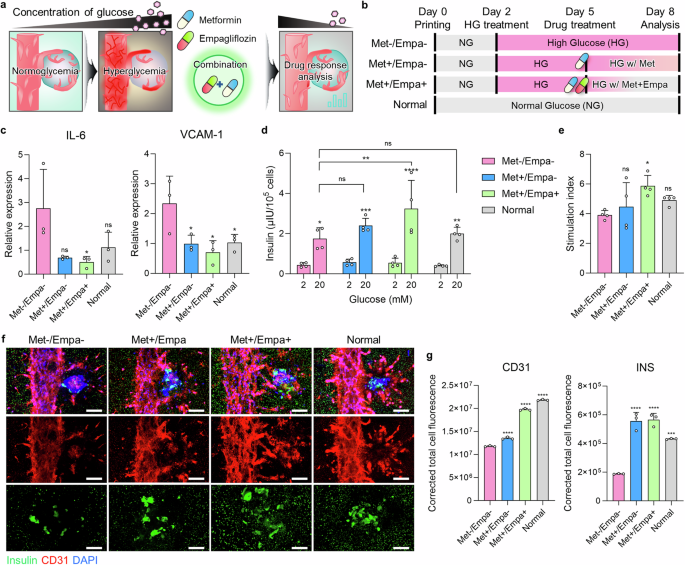
 Main ConclusionsThis study created a customized islet-specific microenvironment through bioprinting technology to promote the maturation of stem cell (SC)-derived islets. In the study, the authors optimized the combination of pancreas-specific extracellular matrix (ECM) and basement membrane proteins, utilizing bioprinting to reproduce the spatial structure surrounding the islets. This bioprinted islet-specific microenvironment facilitated the coordination between islets and blood vessels, supporting structural and functional characteristics similar to natural islets. The study indicates that this strategy not only improves the functionality of SC-derived islets but also provides significant potential for research on islet development, maturation, and diabetes disease modeling, with broad prospects for translational applications in the future.
Main ConclusionsThis study created a customized islet-specific microenvironment through bioprinting technology to promote the maturation of stem cell (SC)-derived islets. In the study, the authors optimized the combination of pancreas-specific extracellular matrix (ECM) and basement membrane proteins, utilizing bioprinting to reproduce the spatial structure surrounding the islets. This bioprinted islet-specific microenvironment facilitated the coordination between islets and blood vessels, supporting structural and functional characteristics similar to natural islets. The study indicates that this strategy not only improves the functionality of SC-derived islets but also provides significant potential for research on islet development, maturation, and diabetes disease modeling, with broad prospects for translational applications in the future. Discussion SummaryThis study proposes a synergistic approach combining a customized islet microenvironment based on pancreatic ECM with bioprinting technology to achieve functional coordination between SC-derived islets, ECM, and vascular networks. By optimizing the in vitro pancreatic microenvironment, the research successfully reproduced the inherent complex components of pancreatic tissue to address the inevitable reduction of basement membrane proteins during decellularization. Based on bioprinting technology, particularly bath bioprinting, the study achieved strategic arrangements of HICA-V to reproduce the three-dimensional pancreas-specific geometric structures. The research also demonstrated that HICA-V can simulate functional coordination in vivo, including physiological responses of SC-derived islets and vascular networks under healthy and inflammatory conditions. This engineering approach provides a promising pathway for studying how environmental factors influence islet development, maturation, and diabetes disease modeling, enhancing the potential for translating diabetes treatment into clinical applications.Note: This public account only interprets academic literature and provides no guidance or advice.
Discussion SummaryThis study proposes a synergistic approach combining a customized islet microenvironment based on pancreatic ECM with bioprinting technology to achieve functional coordination between SC-derived islets, ECM, and vascular networks. By optimizing the in vitro pancreatic microenvironment, the research successfully reproduced the inherent complex components of pancreatic tissue to address the inevitable reduction of basement membrane proteins during decellularization. Based on bioprinting technology, particularly bath bioprinting, the study achieved strategic arrangements of HICA-V to reproduce the three-dimensional pancreas-specific geometric structures. The research also demonstrated that HICA-V can simulate functional coordination in vivo, including physiological responses of SC-derived islets and vascular networks under healthy and inflammatory conditions. This engineering approach provides a promising pathway for studying how environmental factors influence islet development, maturation, and diabetes disease modeling, enhancing the potential for translating diabetes treatment into clinical applications.Note: This public account only interprets academic literature and provides no guidance or advice.
END