
Inspired by the clinical enhancement of radiotherapy efficacy through hyperthermia, this study demonstrates a pure organic nanoparticle’s multimodal diagnostic application in tumor combination therapy, integrating imaging and photothermal properties. This nanoparticle possesses various unique characteristics and functions, including excellent photostability and thermal stability, strong fluorescence emission in the near-infrared II (NIR-II) region, extremely high photothermal conversion efficiency, good biocompatibility, significant radiotherapy sensitization performance, and effective tumor site enrichment capability. In vitro and in vivo experimental results indicate that this nanomaterial is highly suitable as a synergistic photothermal radiotherapy platform guided by NIR-II fluorescence, NIR-I photoacoustic, and photothermal tri-modal imaging. During radiotherapy, this material acts as a radiosensitizer to alleviate the tumor hypoxic microenvironment, regulate the cell cycle, and induce apoptosis and immunogenic cell death, demonstrating its potential application in clinical tumor treatment.
Research Background
Radiotherapy is a conventional strategy for treating various cancers, inducing cell death through ionizing radiation that causes DNA double-strand breaks. However, the DNA free radicals generated by ionizing radiation require the stable presence of oxygen molecules to exert effective destructive effects, highlighting the critical role of oxygen in radiosensitization. The hypoxic state of the tumor microenvironment has become one of the main factors leading to radiotherapy resistance, stemming from the high metabolic demand of tumor tissues for oxygen and the insufficient oxygen supply caused by abnormal tumor vascular structures. Under hypoxic conditions, DNA free radicals are easily reduced by thiol-containing compounds, thereby weakening DNA damage, while activating signaling pathways such as HIF-1α and VEGF, promoting the generation of abnormal blood vessels, which increases the risk of tumor recurrence and reduces the efficacy of radiotherapy. Consequently, radiotherapy resistance forces clinicians to employ multiple or high-dose irradiations to enhance treatment effects.
Photothermal technology has broad application prospects in modern medicine, covering areas such as smart drug delivery, anti-tumor therapy, anti-infection treatment, and neural regulation. Photothermal therapy (PTT) relies on the conversion of light energy into heat to induce localized thermal damage; when the local temperature of the tumor rises to supraphysiological levels, the DNA repair mechanisms of tumor cells are inhibited, and blood flow and oxygenation levels are disturbed, thereby helping to alleviate hypoxic conditions. Additionally, PTT can induce cell cycle arrest at the division phase sensitive to radiotherapy and release a series of damage-associated molecular patterns (DAMPs), such as high mobility group box 1 (HMGB1), calreticulin (CRT), and adenosine triphosphate (ATP), enhancing immune cell infiltration into the tumor and inducing immunogenic cell death. The near-infrared region has gained widespread attention in photothermal therapy due to its superior tissue penetration capabilities. However, the uneven heat distribution within tumor tissues, especially in the peripheral regions near large blood vessels, leads to heat dissipation, making single thermal therapy insufficient for complete tumor eradication and increasing the risk of recurrence. Therefore, combining other therapeutic modalities to enhance efficacy while minimizing damage to surrounding tissues has become necessary.
Currently, multimodal integrated photothermal diagnostic and therapeutic platforms are rapidly developing, capable of integrating fluorescence imaging, photothermal imaging, photoacoustic imaging, photothermal therapy, and photodynamic therapy into a single system. This strategy is considered an important supplement to traditional tumor treatment methods due to its real-time visualization, high selectivity, non-invasiveness, low resistance, and good therapeutic effects, providing new ideas for enhancing the precision of diagnosis and treatment.
Various photosensitizers have been used for photothermal or photodynamic therapy, including inorganic nanomaterials, metal-organic frameworks, semiconductor polymer nanoparticles, and pure organic molecules. Although many photothermal agents have been explored for radiosensitization therapy, most are based on inorganic or metallic materials, which have significant disadvantages in terms of biotoxicity and synthetic complexity, limiting their clinical translation. In contrast, pure organic molecules offer advantages such as clear structure, functional modification, and good biocompatibility. However, research on their radiosensitization is relatively scarce. Traditional organic photosensitizers often suffer from aggregation-caused quenching (ACQ), small Stokes shifts, and poor photostability, limiting their practical applications. Aggregation-induced emission (AIE) characteristic organic photosensitizers can effectively circumvent these defects, exhibiting superior photothermal conversion efficiency. AIE is a special photophysical phenomenon referring to a class of molecular systems that emit weakly or not at all in a dissolved state but exhibit enhanced emission in an aggregated state. Inspired by the clinical hyperthermia enhancing radiotherapy effects, it is speculated that photothermal therapy can also enhance the sensitivity of tumor tissues to radiotherapy, achieving efficient tumor killing, reducing damage to surrounding tissues, and lowering radiation doses. Therefore, the combination of photothermal and radiotherapy is expected to play a synergistic role in tumor treatment. However, research on pure organic nanoparticles combined with PTT and RT is still relatively scarce.
Based on previous research, a pure organic nanoparticle system (2TT-oC6B NPs) was designed and constructed with AIE luminescent molecule 2TT-oC6B as the core for combined photothermal and radiotherapy tumor treatment. This nanoparticle exhibits superior stability, photothermal conversion efficiency, biocompatibility, and near-infrared absorption and emission properties. It can generate sufficient heat under low-power 808 nm laser irradiation. The laser-activated 2TT-oC6B NPs can regulate the tumor cell cycle, induce apoptosis, and improve the hypoxic microenvironment of tumor tissues, significantly enhancing the therapeutic effect of radiotherapy. Ultimately, this nanoparticle achieves excellent anti-tumor effects through the synergistic action of photothermal and radiotherapy.
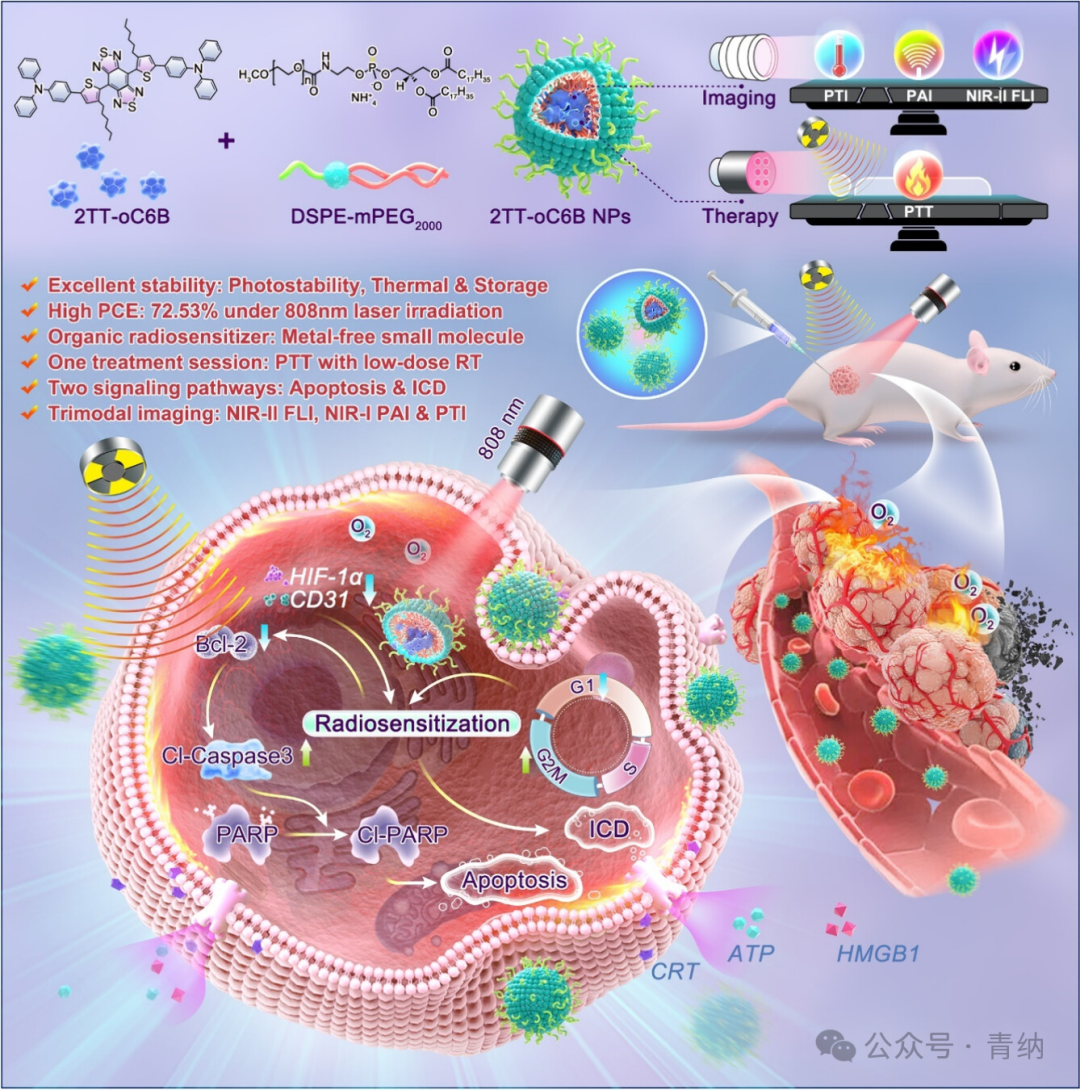
Schematic diagram. Design of 2TT-oC6B nanoparticles and its mechanism of promoting low-dose radiotherapy based on single photothermal therapy.
Research Results
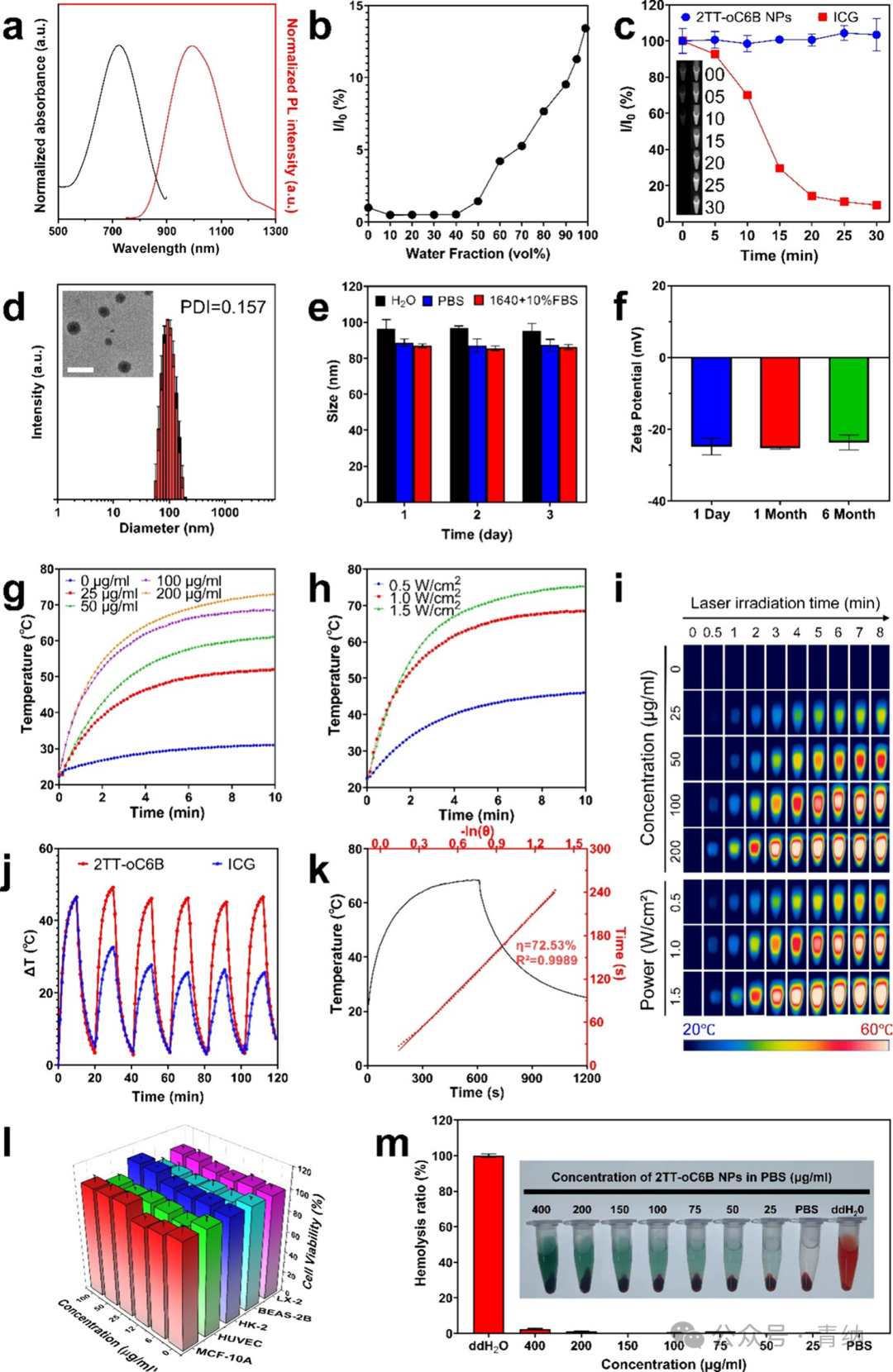
Figure 1. Characterization and performance of 2TT-oC6B NPs. (a) Absorption and emission spectra of 2TT-oC6B NPs. (b) AIE curve as a function of water volume fraction. (c) Photostability curves of 2TT-oC6B NPs and ICG under continuous laser irradiation (808 nm, 1 W/cm², 30 min). Insets show NIR-II fluorescence images of 2TT-oC6B NPs (left) and ICG (right) at different time points. (d) Particle size distribution of 2TT-oC6B NPs in water and their transmission electron microscopy image (inset). Scale bar: 200 nm. (e) Stability analysis of 2TT-oC6B NPs in H₂O, PBS, and RPMI 1640 + 10% FBS at 25 °C. (f) Zeta potential of 2TT-oC6B NPs at different time points. (g) Photothermal time–temperature curves under different concentration conditions. (h) Photothermal time–temperature curves under different laser powers. (i) Results recorded by thermal imaging camera. (j) Heating curves of photothermal stability tests (6 cycles of laser on/off, 1 W/cm²). (k) Photothermal conversion efficiency (PCE) heating curves and calculated results. (l) Cell viability of MCF-10A, HUVEC, HK-2, BEAS-2B, and LX-2 cells after 24 hours of incubation with different concentrations of 2TT-oC6B NPs. (m) Hemolysis test results of 2TT-oC6B NPs.

Figure 2. In vitro cell experiments using 2TT-oC6B NPs for PTT combined with RT. (a) CLSM images of 4T1 cells stained with FITC-2TT-oC6B NPs and LysoTracker Red. Scale bar: 10 μm. (b) Cell viability of 4T1 cells after treatment with different concentrations of 2TT-oC6B NPs under NIR irradiation and/or RT conditions. (c) CLSM images of 4T1 cells after treatment with different concentrations of 2TT-oC6B NPs under NIR irradiation and/or RT conditions. Scale bar: 100 μm. (d) Qualitative images of γ-H2AX foci formation. Scale bar: 10 μm. (e) Photographs of colony formation experiments. (NPs: 2TT-oC6B NPs; NIR: 808 nm, 1 W/cm²; RT: 4 Gy)
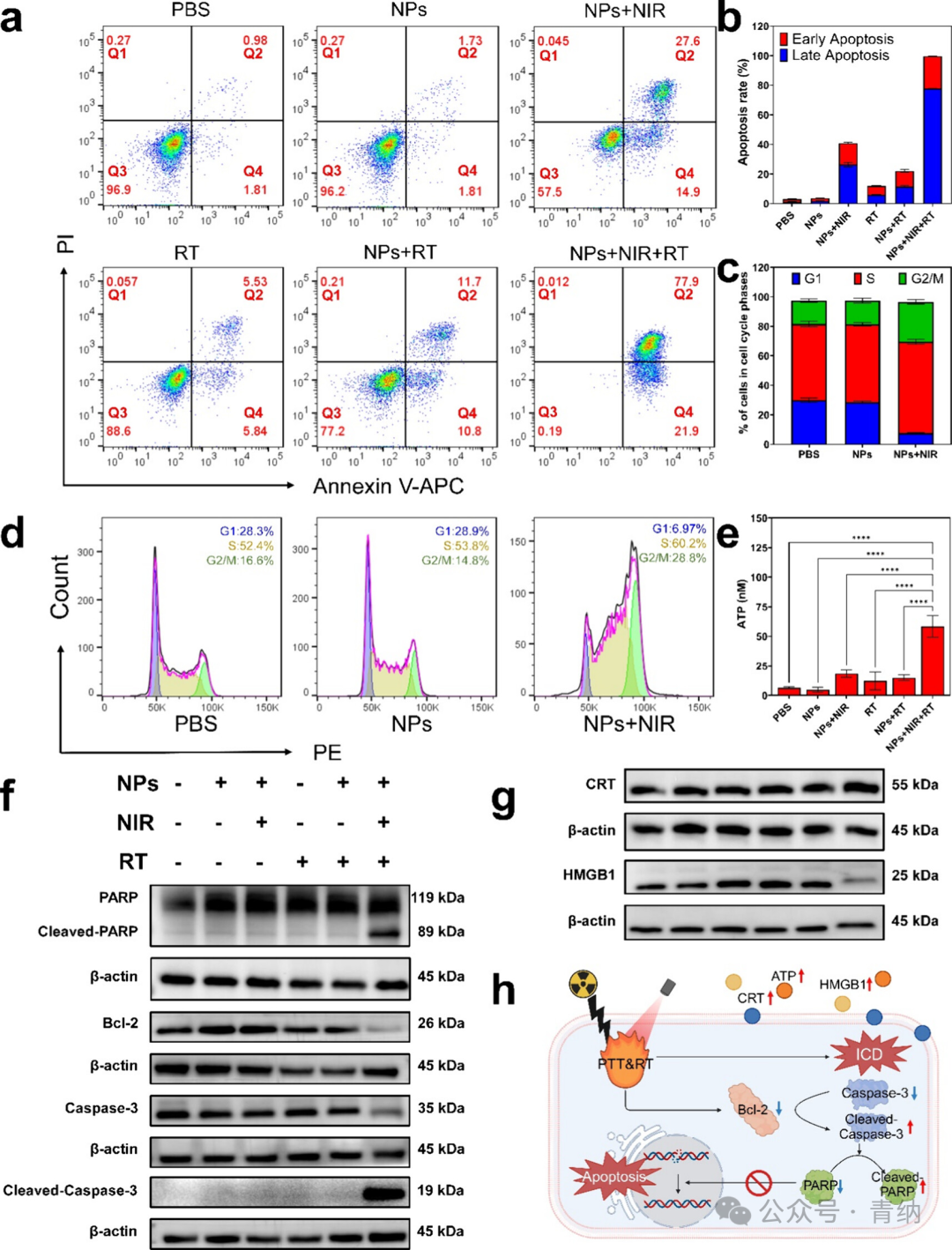
Figure 3. Efficacy and mechanism of PTT combined with RT at the cellular level. (a) Flow cytometry apoptosis analysis of 4T1 cells after different treatments. Q1: Proportion of necrotic cells (PI/APC, ±); Q2: Proportion of late apoptotic cells (PI/APC, +/+); Q3: Proportion of live cells (PI/APC, −/−); Q4: Proportion of early apoptotic cells (PI/APC, ∓). (b) Quantitative analysis of the percentage of apoptotic cells in (a). (c) Quantitative analysis of the cell cycle proportions in (d). (d) Flow cytometry cell cycle analysis of 4T1 cells after different treatments. (e) Quantitative analysis of extracellular ATP content in 4T1 cells after different treatments. (f) Western blot analysis of apoptosis-related proteins. (g) Western blot analysis of ICD-related proteins. (h) Schematic diagram of the molecular mechanism of cell killing under laser irradiation (created with BioRender.com). (NPs: 2TT-oC6B NPs; NIR: 808 nm, 1 W/cm²; RT: 4 Gy)
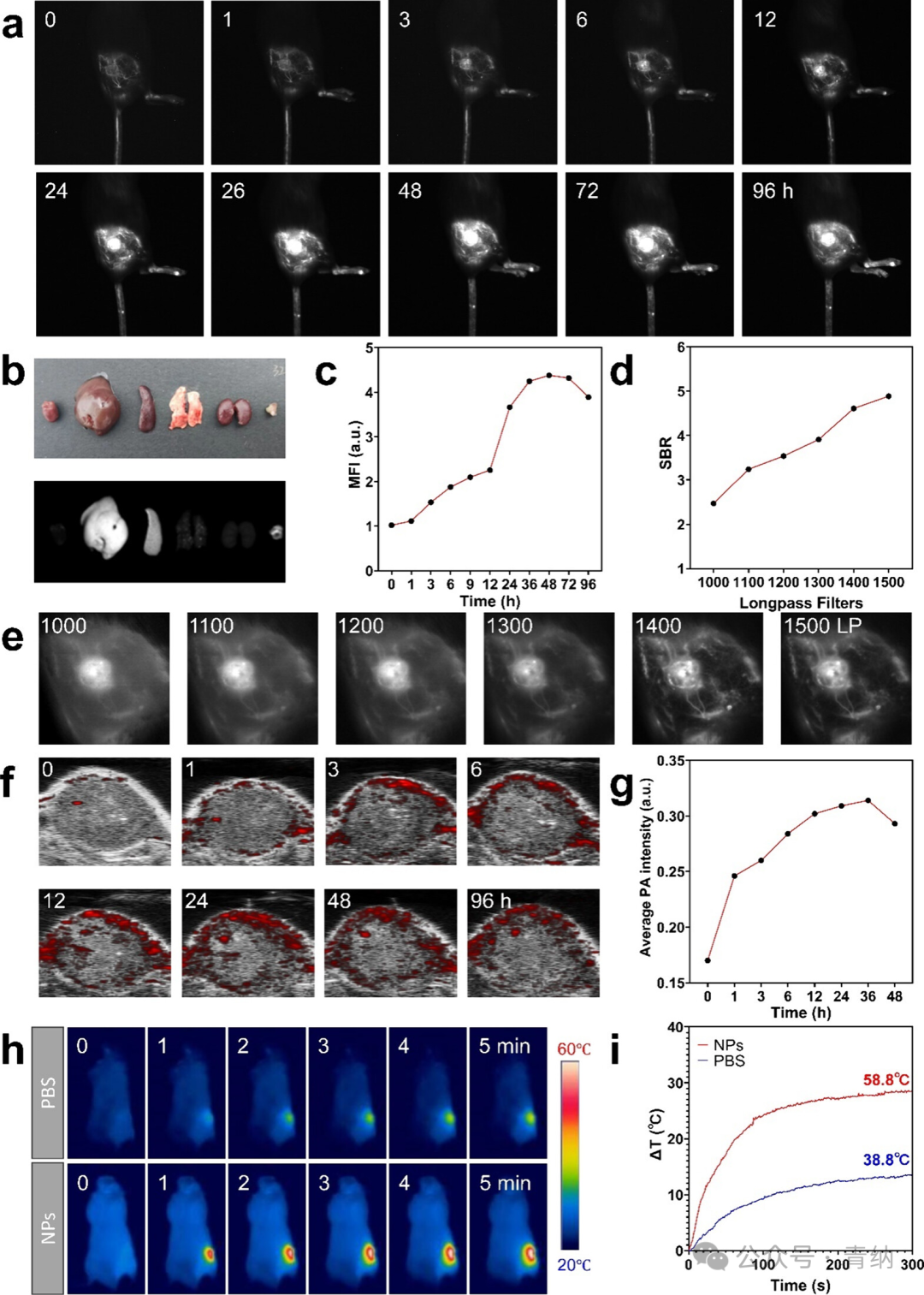
Figure 4. In vivo multimodal imaging performance of 2TT-oC6B NPs in BALB/c mice bearing 4T1 tumors. (a) NIR-II fluorescence imaging of tumor tissues at different time points after intravenous injection of 2TT-oC6B NPs (1 mg/mL, 200 μL). (b) Ex vivo NIR-II fluorescence images and bright field images of tumors and major organs 96 hours post-injection. (c) Average NIR-II fluorescence intensity of tumor tissues at different time points in (a). (d) Signal-to-noise ratio changes under different long-pass filters (LP filter) at 9 h in (e). (e) NIR-II fluorescence imaging of tumor tissues at 9 h using different LP filters. (f) Photoacoustic imaging (PAI) images of tumor tissues at different time points after intravenous injection of 2TT-oC6B NPs (1 mg/mL, 200 μL). (g) Average PA signal intensity of tumors at different time points in (f). (h) Photothermal imaging (PTI) images of tumor tissues under continuous laser irradiation (808 nm, 1 W/cm², 0–5 min). (i) Real-time temperature change curve of tumor tissues in (h).
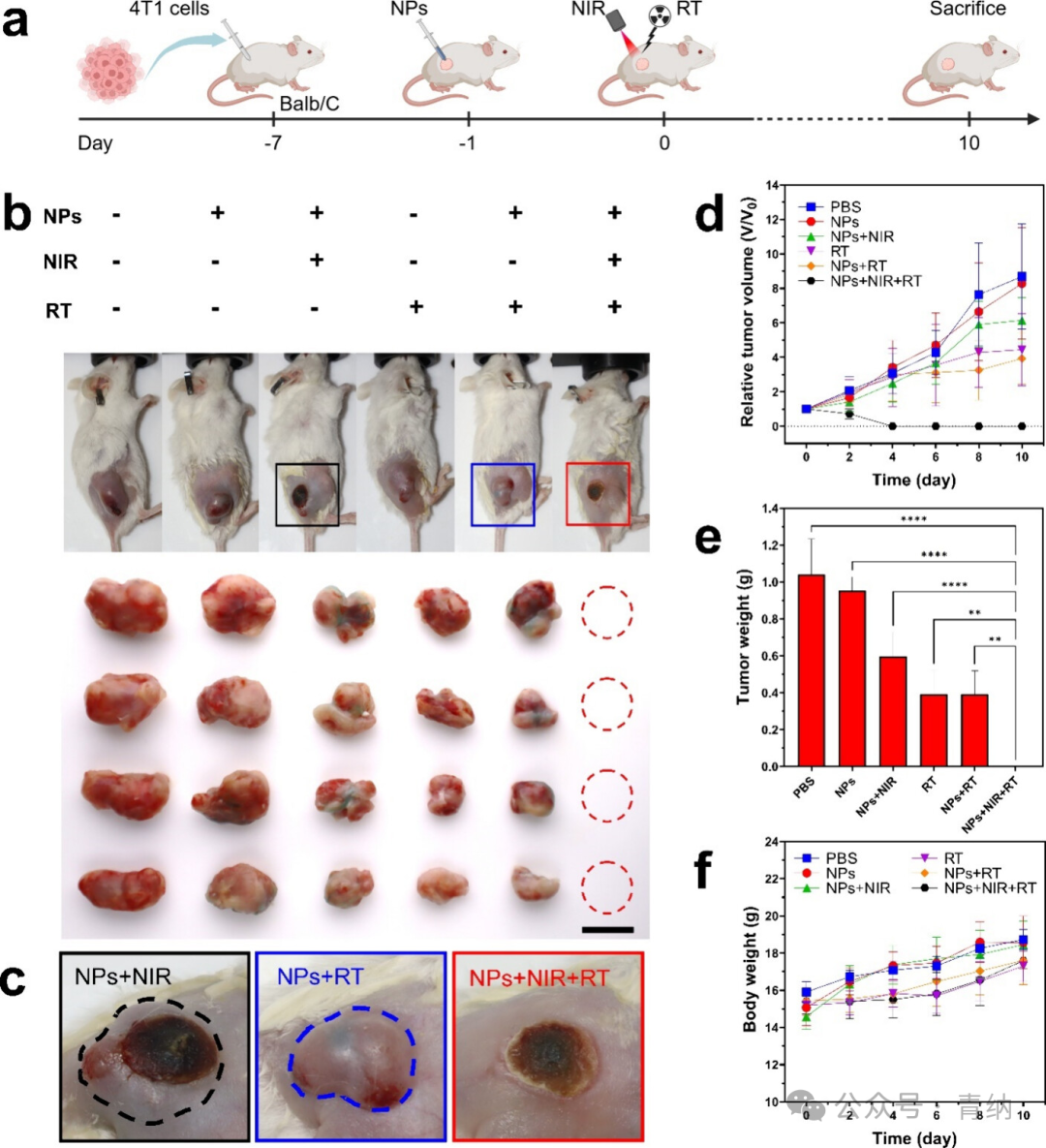
Figure 5. In vivo anti-tumor effects of 2TT-oC6B NPs in BALB/c mice bearing 4T1 tumors. (a) Flowchart of the treatment process for synergistic PTT-RT therapy using 2TT-oC6B NPs (created with BioRender.com). (b) Photographs of mice and tumors on day 10 after different treatments. Scale bar: 10 mm. (c) Images of tumor regions on day 10 after treatment with or without 2TT-oC6B NPs combined with NIR and/or RT (dashed circles indicate tumor recurrence areas). (d) Growth curves of subcutaneous 4T1 tumors in different treatment groups. (e) Tumor weights in different treatment groups. (f) Weight changes of subcutaneous 4T1 tumor-bearing mice in different treatment groups. (NIR: 808 nm, 1 W/cm²; RT: 4 Gy)
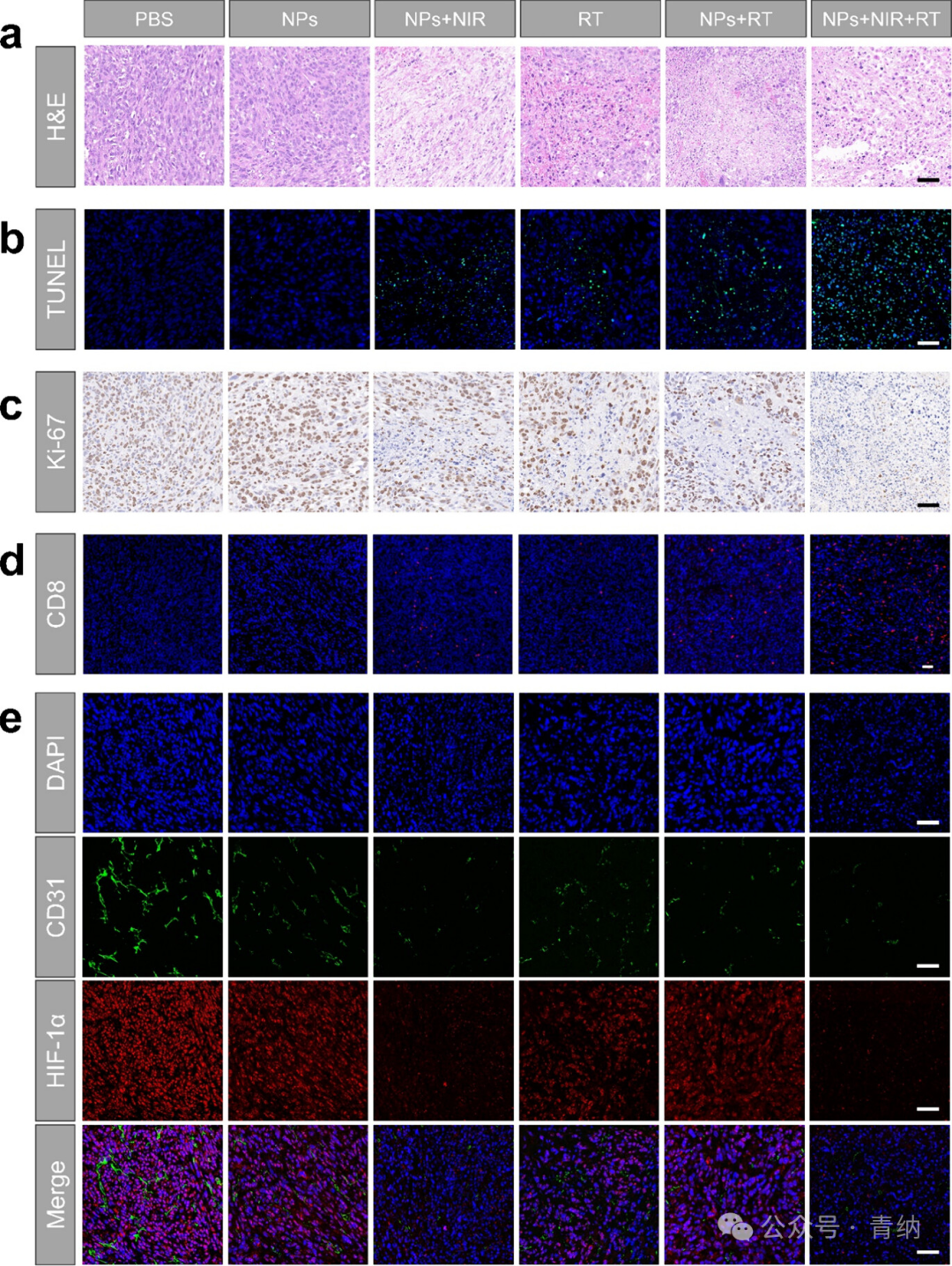
Figure 6. Efficacy and mechanism of PTT combined with RT at the tissue level in vivo. (a–c) HE staining, TUNEL staining, and Ki-67 staining analysis of tumor tissues after different treatments. Scale bar: 50 μm. (d) Immunofluorescence images of infiltrating CD8⁺ T cells in tumor tissues. Scale bar: 50 μm. (e) Immunofluorescence images of HIF-1α and CD31 in tumor tissues. Scale bar: 50 μm.