
Summary of This Issue:
-
Vitamin C (ascorbic acid) is an important antioxidant that we all need. -
Various foods are rich in Vitamin C, but its dietary sources are mainlyplant-based foods. -
Humans have lost the ability to synthesize Vitamin C, which may bring other advantages.
This article has 2667 words, writing time: 5 hours, recommended reading time: 4 minutes.
Vitamin C: Miracle Drug or Philosopher’s Stone?
The True Identity of Vitamin C
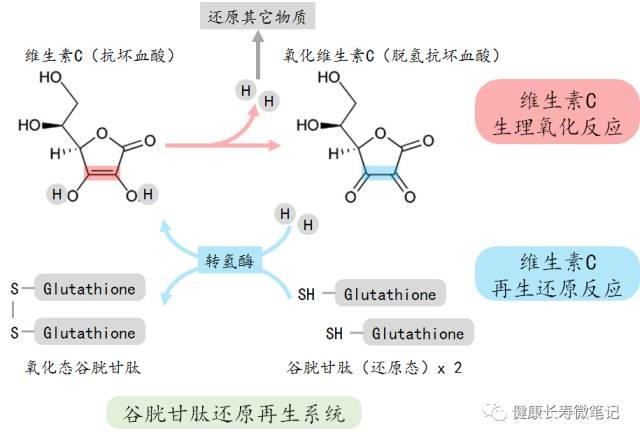

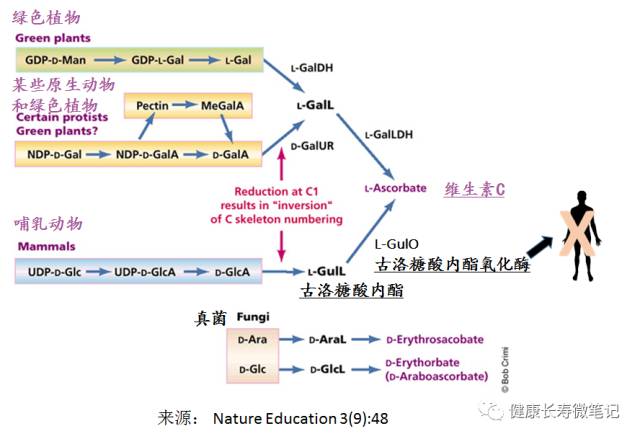
The Biosynthesis of Vitamin C
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Humans and Vitamin C: The Loss of Synthesis
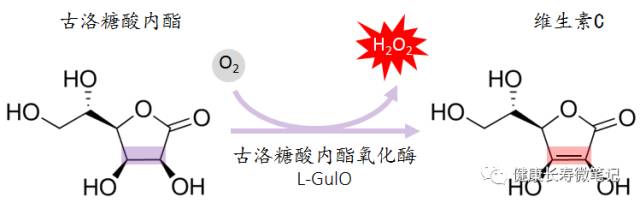
-
In clinical studies, it was found that taking 500 mg of Vitamin C daily can significantly reduce uric acid levels in the blood. -
However, some studies have found that using the same dose of Vitamin C can lower blood uric acid, but the extent is not sufficient to alleviate gout symptoms. -
Other studies reported that in patients undergoing kidney dialysis, the higher the uric acid level in the blood, the lower the amount of oxidized Vitamin C.
References:
https://www.ncbi.nlm.nih.gov/pubmed/21671418 http://www.wiley.com/WileyCDA/PressRelease/pressReleaseId-108712.html https://www.ncbi.nlm.nih.gov/pubmed/11239034