 Click the blue text above“ioncology” to follow us, then click the “…” menu in the upper right corner and select “Set as Favorite”Neoadjuvant concurrent chemoradiotherapy remains the standard treatment for locally advanced esophageal squamous cell carcinoma (ESCC). Recent studies have also shown survival advantages from neoadjuvant chemotherapy alone, but the improvement in pathological complete response (pCR) rates with neoadjuvant chemotherapy alone is limited. A multicenter, randomized, double-blind phase II study led by Yong Li from the Thoracic Surgery Department of the Cancer Hospital of the Chinese Academy of Medical Sciences evaluated the safety and efficacy of a novel PD-L1 inhibitor, Socazolimab (also known as Toripalimab), in combination with albumin-bound paclitaxel/cisplatin for neoadjuvant treatment of locally advanced ESCC. The results were presented in poster form at the 2022 European Society for Medical Oncology (ESMO 2022) Annual Meeting (Abstract 1207P) (First author: Yong Li, Corresponding author: Gao Shugang).
Click the blue text above“ioncology” to follow us, then click the “…” menu in the upper right corner and select “Set as Favorite”Neoadjuvant concurrent chemoradiotherapy remains the standard treatment for locally advanced esophageal squamous cell carcinoma (ESCC). Recent studies have also shown survival advantages from neoadjuvant chemotherapy alone, but the improvement in pathological complete response (pCR) rates with neoadjuvant chemotherapy alone is limited. A multicenter, randomized, double-blind phase II study led by Yong Li from the Thoracic Surgery Department of the Cancer Hospital of the Chinese Academy of Medical Sciences evaluated the safety and efficacy of a novel PD-L1 inhibitor, Socazolimab (also known as Toripalimab), in combination with albumin-bound paclitaxel/cisplatin for neoadjuvant treatment of locally advanced ESCC. The results were presented in poster form at the 2022 European Society for Medical Oncology (ESMO 2022) Annual Meeting (Abstract 1207P) (First author: Yong Li, Corresponding author: Gao Shugang). Patients with cT2N+M0 or cT3-4aN+/-M0 ESCC were included in the study. After the phase IB safety assessment, patients were randomly assigned in a 1:1 ratio in phase II to receive either Socazolimab (5 mg/kg IV on Day 1, Group S) or placebo (Group P), along with albumin-bound paclitaxel (125 mg/m2 IV on Days 1/8) and cisplatin (75 mg/m2 IV on Day 1), repeated every 21 days for a total of 4 cycles. Patients in Group S with pathological residual disease post-surgery continued Socazolimab treatment for 12 cycles. The primary endpoint was major pathological response (MPR), and secondary endpoints included pCR, R0 resection rate, event-free survival (EFS), overall survival (OS), and safety.
Patients with cT2N+M0 or cT3-4aN+/-M0 ESCC were included in the study. After the phase IB safety assessment, patients were randomly assigned in a 1:1 ratio in phase II to receive either Socazolimab (5 mg/kg IV on Day 1, Group S) or placebo (Group P), along with albumin-bound paclitaxel (125 mg/m2 IV on Days 1/8) and cisplatin (75 mg/m2 IV on Day 1), repeated every 21 days for a total of 4 cycles. Patients in Group S with pathological residual disease post-surgery continued Socazolimab treatment for 12 cycles. The primary endpoint was major pathological response (MPR), and secondary endpoints included pCR, R0 resection rate, event-free survival (EFS), overall survival (OS), and safety.
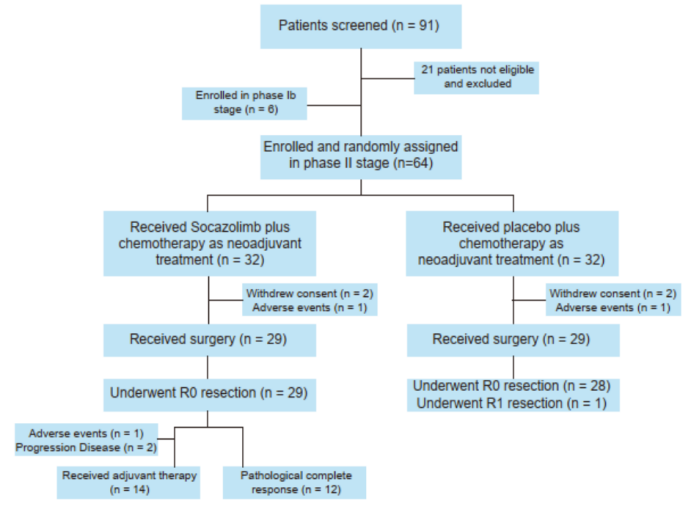
Figure 1. Study Flowchart (Source: ESMO 2022 Conference Poster)Results showed that in phase IB, no dose-limiting toxicities were observed in the first 6 patients. In phase II, 64 patients were assigned to Group S (n=32) and Group P (n=32). The pathological response of patients in Group S and Group P is shown in Figure 2.
-
As of January 5, 2022, 29 patients (90.6%) in both groups underwent surgery, with 29 patients (100%) in Group S and 28 patients (98.6%) in Group P undergoing R0 resection.
-
The MPR rates for Group S and Group P were 68.97% and 62.07% (P=0.509), respectively, while the pCR rates were 41.4% and 27.6% (P=0.311).
-
The incidence of ypT0 observed in Group S was significantly higher (37.93% vs. 3.45%; P=0.001). The downstaging of T and N stages in patients from Group S and Group P after neoadjuvant treatment is shown in Figure 3.
-
The data for EFS and OS are still immature.
-
The incidence of treatment-related adverse events (AEs) of grade 3 or higher was 65.5% and 62.5%, respectively. The most common AEs were neutropenia (59.4% vs. 56.3%), leukopenia (43.8% vs. 25.0%), and hypokalemia (18.8% vs. 0). Each group had 2 patients with serious surgical complications.

Figure 2. Comparison of Pathological Responses between Socazolimab+TP Group and Placebo+TP Group (TP: Albumin-bound Paclitaxel/Cisplatin) (Source: ESMO 2022 Conference Poster)
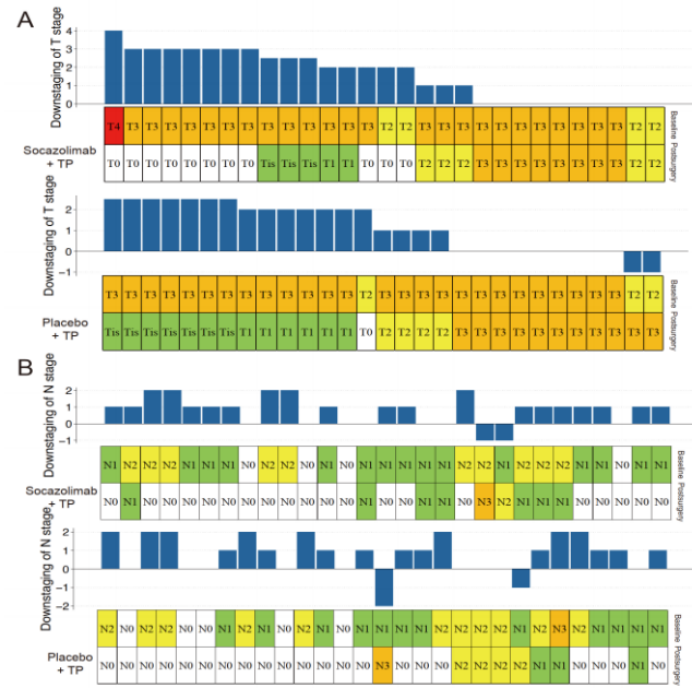
Figure 3. Downstaging of T (A) and N (B) Stages after Neoadjuvant Treatment in Socazolimab+TP Group and Placebo+TP Group (TP: Albumin-bound Paclitaxel/Cisplatin)
(Source: ESMO 2022 Conference Poster)The study concluded that although there were no statistical differences between the two groups, neoadjuvant Socazolimab combined with chemotherapy showed higher MPR and pCR rates in locally advanced ESCC without increasing perioperative complications. This strategy needs further validation in phase III trials.
Source
Yong Li, et al. Neoadjuvant PD-L1 inhibitor (socazolimab) plus chemotherapy in patients with locally advanced esophageal squamous cell carcinoma (ESCC): A multicenter, randomized, double-blind phase II study. Presented at: the European Society for Medical Oncology (ESMO) World Congress on Oesophagogastric cancer in Paris, 9-13 September 2022. Abstract 1207P.
END

(Source: Tumor Outlook – Digestive News)
Disclaimer
All original articles are copyrighted by “Tumor Outlook”. Sharing and reproduction are welcome. This article is intended for medical and health professionals to understand the latest pharmaceutical information and does not represent the views of this platform. Such information cannot replace professional medical guidance and should not be considered as medical advice. If this information is used for purposes other than information, the site and the author bear no related responsibility.
