Extraction, Purification, and Gastroprotective Activity of Glucomannan from Bletilla Striata
Presenter: First Year Graduate Student Di Zhenning
This study extracted and purified a water-soluble polysaccharide (BSP) from the pseudobulb of Bletilla striata and analyzed its preliminary structure and gastroprotective activity. The results showed that BSP is a glucomannan with a molar ratio of 7.45:2.55 (Man:Glc) and a molecular weight of approximately 1.7×105 Da. BSP exhibited significant protective effects against ethanol-induced damage in GES-1 cells in vitro and demonstrated good gastroprotective effects in vivo, especially at high doses of BSP (100 mg/kg), which reduced gastric mucosal ulcer index and increased the ulcer inhibition rate. The mechanism may be related to enhancing the antioxidant capacity of gastric tissues and inhibiting apoptosis pathways. Moreover, BSP showed gastroprotective activity comparable to that of the positive control (omeprazole). In summary, our results suggest that BSP can be regarded as a potential drug for preventing gastric injury.
This article titled “Extraction, Purification, and Determination of the Gastroprotective Activity of Glucomannan from Bletilla Striata” was published in June 2020 by the research team of Niu Junfeng from the College of Life Sciences, Shaanxi Normal University, in the journal Carbohydrate Polymers.

1
Research Background
The gastric secretion of protein hydrolytic enzymes and hydrochloric acid plays an important role in aiding digestion. In daily life, many risk factors such as stress, alcohol, and non-steroidal anti-inflammatory drugs (NSAIDs) can lead to gastric diseases. Currently, many synthetic drugs are widely used as first-line treatments for gastric diseases, including proton pump inhibitors, anticholinergics, histamine receptor antagonists, and antacids, but they can cause many adverse reactions. Therefore, there is a need to develop an efficient and safe gastroprotective drug to address this issue. Extensive research has shown that natural ingredients such as flavonoids, polysaccharides, and polyphenols can exhibit good gastroprotective activity. Among them, Bletilla striata polysaccharide (BSP) has low toxicity and high biological activity, making it a subject of extensive research in the field of phytochemistry. Studies have indicated that BSP can alleviate oxidative stress induced by H2O2 in porcine chondrocytes, which may be related to the antioxidant and anti-inflammatory activities of BSP. Additionally, BSP’s adhesive oral films can promote the healing of acetic acid-induced oral ulcers. However, information regarding the gastroprotective effects of BSP and its protective mechanisms is limited and requires further exploration. This study employed DEAE-52 cellulose and Sephadex G-150 column chromatography to separate and purify BSP, and established in vitro and in vivo ethanol injury models to evaluate its gastroprotective effects.
2
Research Content
Result 1: Extraction and Purification of Bletilla Striata Polysaccharide
The authors extracted 100g of pre-treated powder with 3000 mL of water at 80 °C for 4 hours, then precipitated with 4 times the volume of 95% ethanol at 4 °C for 24 hours. The precipitate was then re-dissolved in distilled water and underwent 10 cycles of freezing and thawing to remove proteins from the solution, followed by dialysis in distilled water for 72 hours to further remove small molecules. Finally, the non-dialyzable liquid was collected and freeze-dried to obtain crude polysaccharide.
Subsequently, the authors re-dissolved the crude polysaccharide in distilled water (10 mg/mL) and loaded it onto a DEAE-52 cellulose column (5.0×60 cm) for chromatography, eluting with a gradient of 0~0.4 M NaCl at a flow rate of 1 mL/min. The total sugar content was determined using the phenol-sulfuric acid method. Finally, the eluent was collected, dialyzed, freeze-dried, and further purified using Sephadex G-150 column (5.0×60 cm). Figure 1 shows a main elution peak (BSP) obtained from DEAE-52 cellulose anion exchange column (Figure 1A) and Sephadex G-150 column chromatography (Figure 1B).
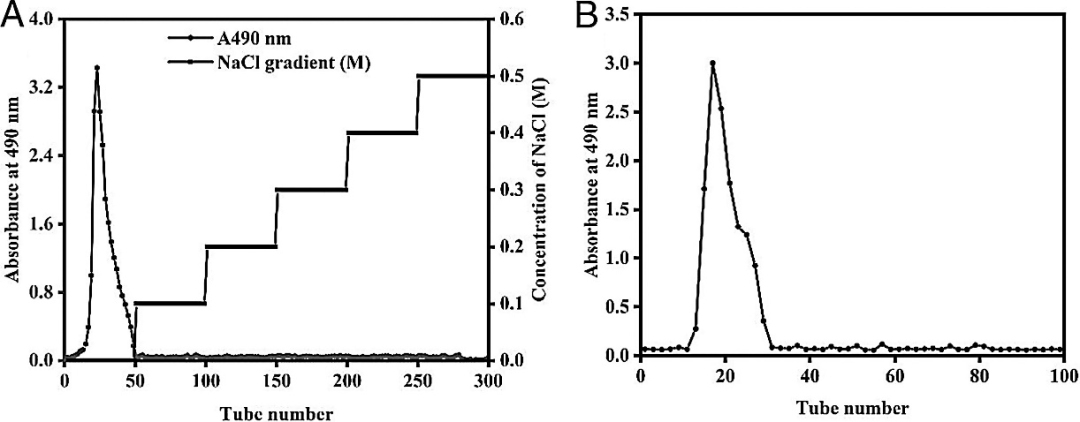
Figure 1: Elution profile of crude polysaccharides from dried Bletilla striata pseudobulbs (A) DEAE-52 cellulose anion exchange chromatography and (B) Sephadex G-150 gel filtration chromatography.
Result 2: Preliminary Characterization of Bletilla Striata Polysaccharide
The authors then conducted some preliminary characterizations of Bletilla striata polysaccharide, including monosaccharide composition, molar mass, and chemical structure. Figure 2 (A-B) shows that BSP consists solely of mannose and glucose in a molar ratio of 7.45:2.55. Many studies have indicated that BSP is a glucomannan. However, their molar percentages vary significantly, which may be caused by various factors such as extraction processes, purification levels, and the source of Bletilla.
Based on Figure 2 (C), the average molecular weight was calculated to be approximately 1.7×105 Da using log Mw= -1.1287t+13.579 (R2=0.9952), with a number-average molecular weight of approximately 1.11×105 Da. Additionally, the polydispersity index (Mw/Mn) of BSP was 1.53. The spectral bands at 323 cm−1 and 2890 cm−1 are considered to be O-H stretching vibrations and C-H asymmetric stretching vibrations, respectively, while the C=O stretching vibration at 1644 cm-1 and the peak at 1250 cm-1 indicate that BSP may be acetylated. The spectral band at 890 cm-1 indicates that Bletilla striata polysaccharide has β-glycosidic bonds, while the bands at 818 cm-1 and 813 cm-1 are due to mannose residues, which are consistent with the results shown in monosaccharide composition.
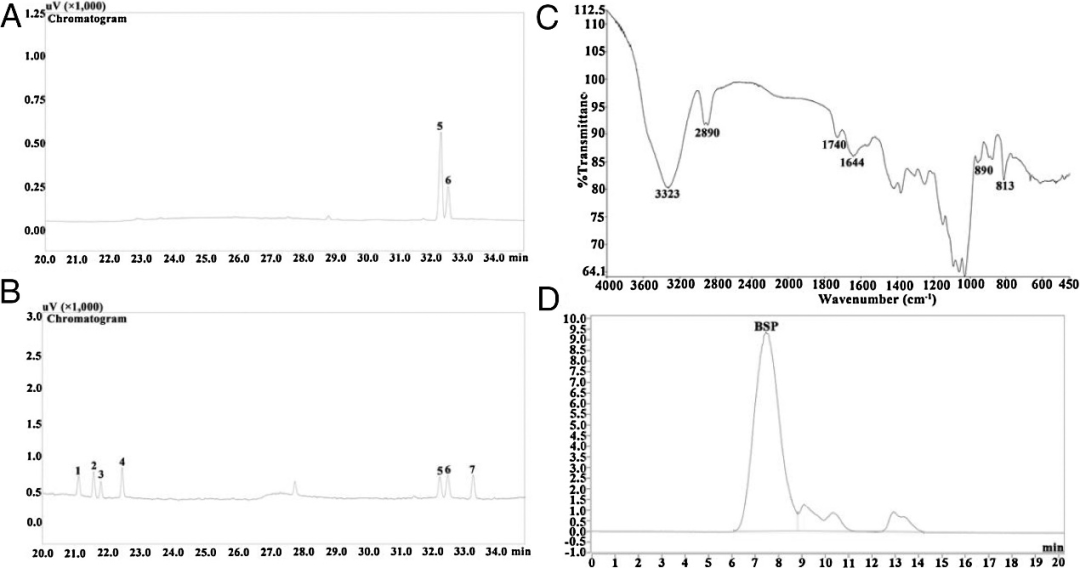
Figure 2 (A) Gas chromatography of BSP and (B) monosaccharide standards (C) Fourier-transform infrared spectroscopy and (D) high-performance liquid chromatography (HPLC) peaks: (1) Rha (2) Fur (3) Ara (4) Xyl (5) Man (6) GLC (7) Gal.
Result 3: In Vitro Gastroprotective Effects of Bletilla Striata Polysaccharide
3.1 Ethanol-Induced Cell Injury Model
The authors cultured GES-1 cells for 24 hours and then incubated the cells in ethanol (50-1600µM) for 3-24 hours, as shown in Figure 3 (A). The results in Figure 3 (B) indicate that cell viability significantly decreased with increasing concentration (100-1600 µM). Furthermore, as the treatment time with ethanol (3−6 h) was extended, cell viability was significantly inhibited. However, after prolonged treatment (12-24 h), cell viability recovered, likely due to the volatility of ethanol and the self-repairing ability of the cells. Notably, after 6 hours of treatment with 100 µM ethanol, cell survival rate was close to 50%. Therefore, this experiment adopted the GES-1 cell injury model induced by ethanol (100 µM, 6 h).
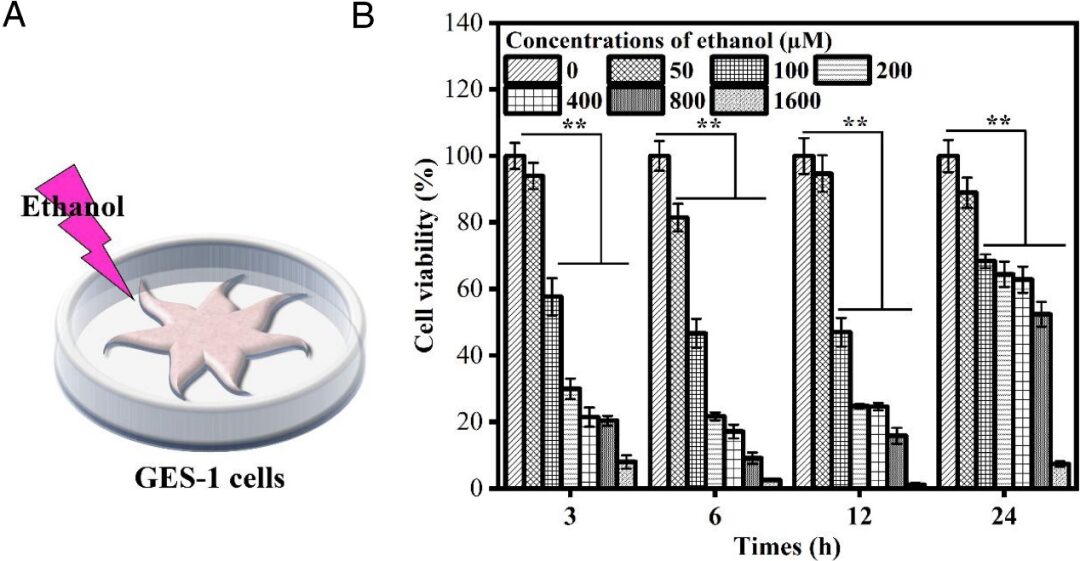
Figure 3 (A) Ethanol-induced GES-1 cell injury model (B) Survival rate of GES-1 cells under different ethanol concentrations and treatment times.
3.2 Study on Cytotoxicity and Gastroprotective Effects of BSP
The authors first measured the cytotoxicity of BSP at different concentrations (0.25-8 mg/mL) on GES-1 cells. Figure 4 (A) shows that BSP only negatively affected cell survival at the highest concentration (8 mg/mL). Therefore, a series of sample concentrations (0.25−4 mg/mL) were selected for further experiments. Based on this, the protective effect of BSP against ethanol-induced GES-1 cell injury was observed. Figure 4 (B) shows that the survival rate of cells in the model group was significantly lower than that of the control group, at 53.8%. However, pre-treatment with BSP significantly increased cell survival rate in a concentration-dependent manner. At a concentration of 4 mg/mL, the cell survival rate significantly increased to 83.8%.
Subsequently, the authors used calmodulin-AM (green fluorescence) staining for live cells and PI (red fluorescence) staining for dead cells to visually observe and analyze the above results. The results in Figure 4 (C) show that cells in the model group exhibited stronger red fluorescence compared to those in the control group, confirming that GES-1 cells underwent ethanol-induced injury. Compared to the model group, the red fluorescence weakened with increasing BSP concentration (0.25−4 mg/mL), indicating significantly enhanced cell protective ability. The authors demonstrated the gastroprotective effect of BSP through in vitro models, while the in vivo gastroprotective effect of BSP in mice requires further investigation.
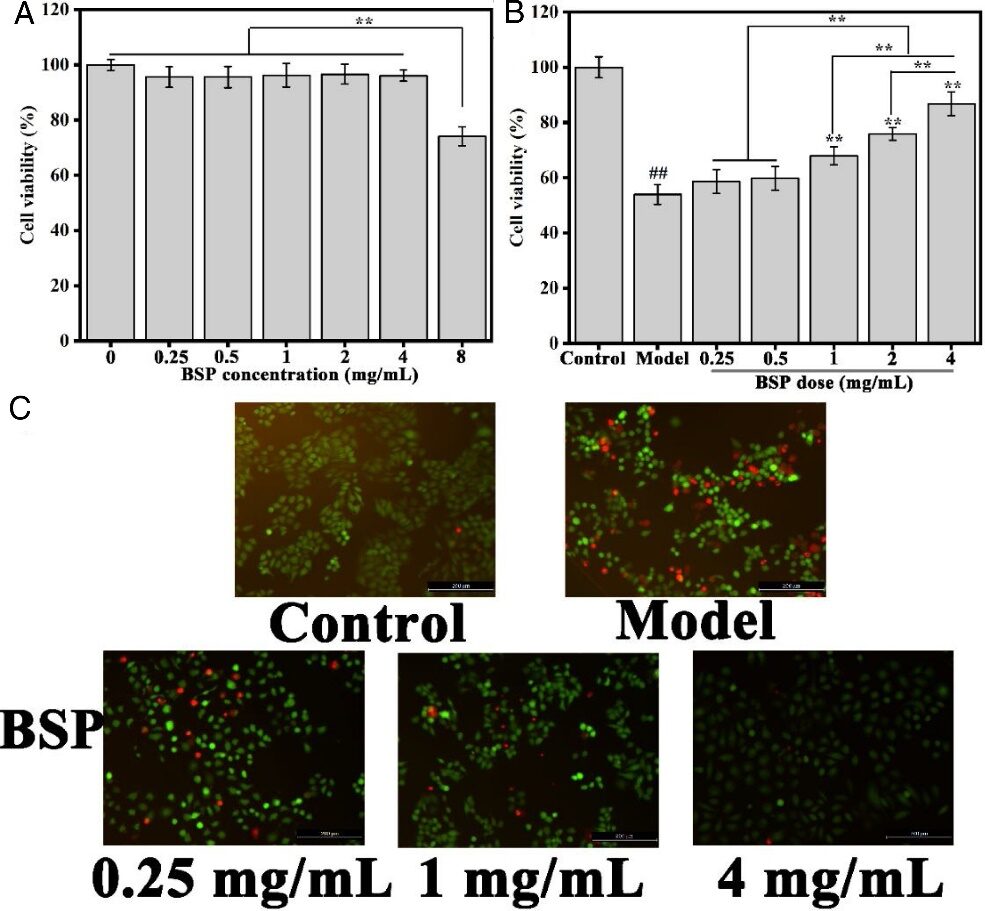
Figure 4 (A) Cytotoxicity (B) Protective effect of BSP on GES-1 cells (C) Live/dead staining of cells after different treatments.
Result 4: Experimental Study on In Vivo Gastroprotective Effects
4.1 Macroscopic Inhibition of Gastric Ulcers
The authors first measured the cytotoxicity of BSP at different concentrations (0.25-8 mg/mL) on GES-1 cells. Excessive alcohol gavage can induce acute gastric injury, characterized by mucosal edema, bleeding, and epithelial cell death. The macroscopic observations of the stomachs of rats in different groups are shown in Figure 5. The stomach mucosa of NC group rats was smooth and without bleeding, while the MC group rats exhibited severe bleeding and edema in the gastric mucosa. The BSP treatment group showed a significant reduction in gastric ulcers, especially in the high dose group (100 mg/kg).
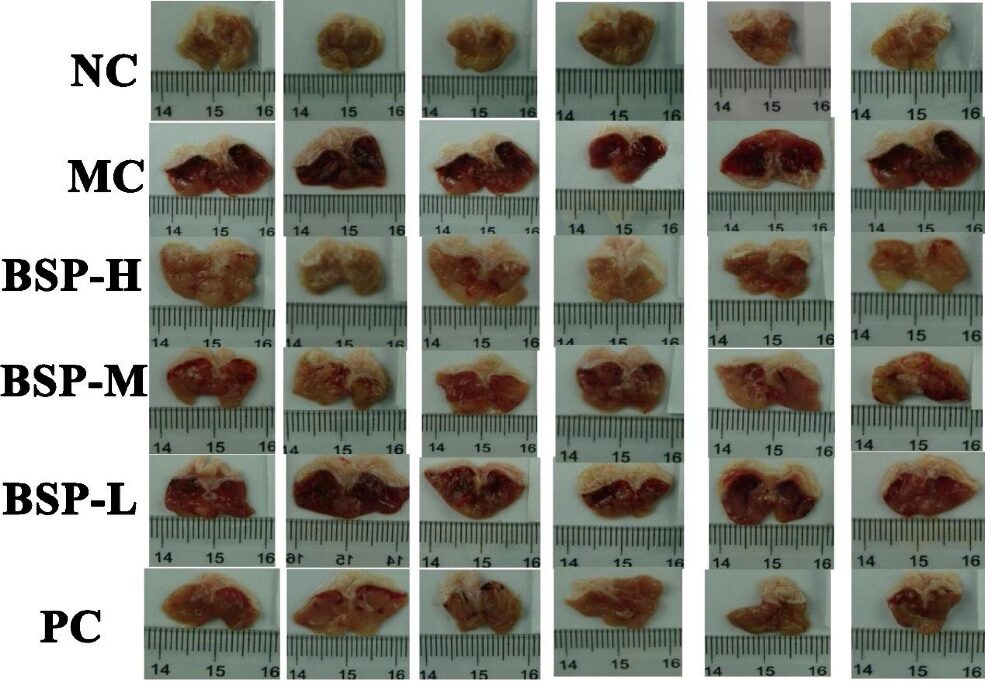
Figure 5: Representative images of gastric tissues from different groups.
4.2 In Vivo Antioxidant Activity
Many literatures indicate that excessive ethanol increases the levels of reactive oxygen species, which attack all biomacromolecules (DNA, proteins, and lipids), ultimately leading to cellular and tissue damage. Compared to the NC group, the antioxidant enzyme activities (SOD, CAT, and GSH-Px) were reduced in the treatment group, but significantly increased compared to the model group, demonstrating a reversal of activity.
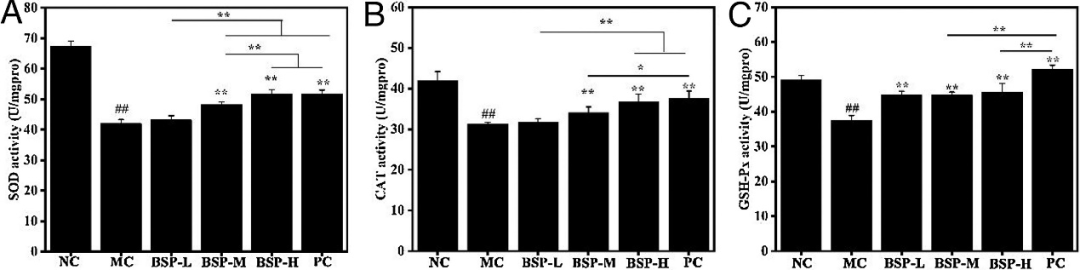
Figure 6: Antioxidant enzyme activities in gastric tissues from different groups: (A) SOD (B) CAT (C) GSH-Px.
Figure 7 shows that an increase in MDA and LPO was observed in the MC group, indicating oxidative stress induced by ethanol. However, after pre-treatment with BSP, the aforementioned phenomena were reversed in a dose-dependent manner. The levels of MDA and LPO were significantly lower than those of the MC group by 38.0% and 19.8%, respectively. Notably, in the high-dose BSP group, the antioxidant enzyme activities were enhanced, and the lipid products were not lower than those produced by the positive drug (omeprazole). These findings suggest that BSP can enhance antioxidant enzyme activities, eliminate free radicals, and inhibit lipid peroxidation.
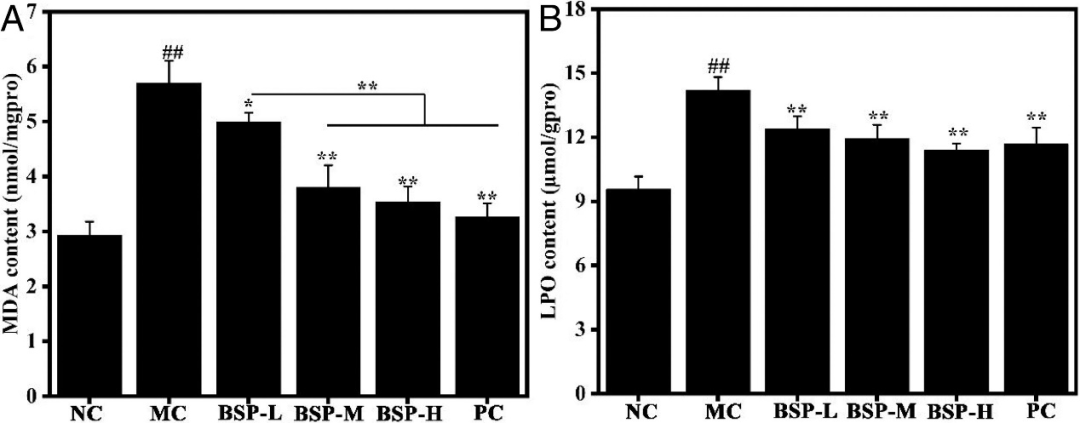
Figure 7: Oxidative products in gastric tissues from different groups (A) MDA and (B) LPO.
4.3 Histopathological Results
The H&E staining results in Figure 8 (A) show that the gastric mucosa structure of the NC group was intact, with no abnormal epithelial necrosis. The gastric mucosa of the MC group rats was severely damaged, with destruction of surface epithelial cells and disordered gastric mucosa structure, along with infiltration of inflammatory cells. However, pre-treatment with BSP or omeprazole alleviated gastric damage, reduced inflammatory cell infiltration, and protected the gastric mucosa structure. These results indicate that BSP possesses activity similar to that of omeprazole. Figure 8 (B) shows a large number of apoptotic cells in the MC group. In contrast, the green fluorescence in BSP-L, BSP-M, BSP-H, and PC groups dimmed, indicating reduced apoptosis of gastric tissue cells. Correspondingly, the apoptosis index was also calculated based on the number of apoptotic cells in the microscopic sections (Figure 8C). The apoptosis index was 2.7±3.0%, 36.4±2.1%, 32.3±2.4%, 28.9±2.6%, 20.4±3.2%, and 16.7±3.9% for NC group, MC group, BSP-L group, BSP-M group, BSP-H group, and PC group, respectively. Interestingly, no significant difference was observed between the high-dose BSP group and the PC group. This suggests that Bletilla striata polysaccharide may alleviate alcoholic gastric ulcers by reducing cell apoptosis.
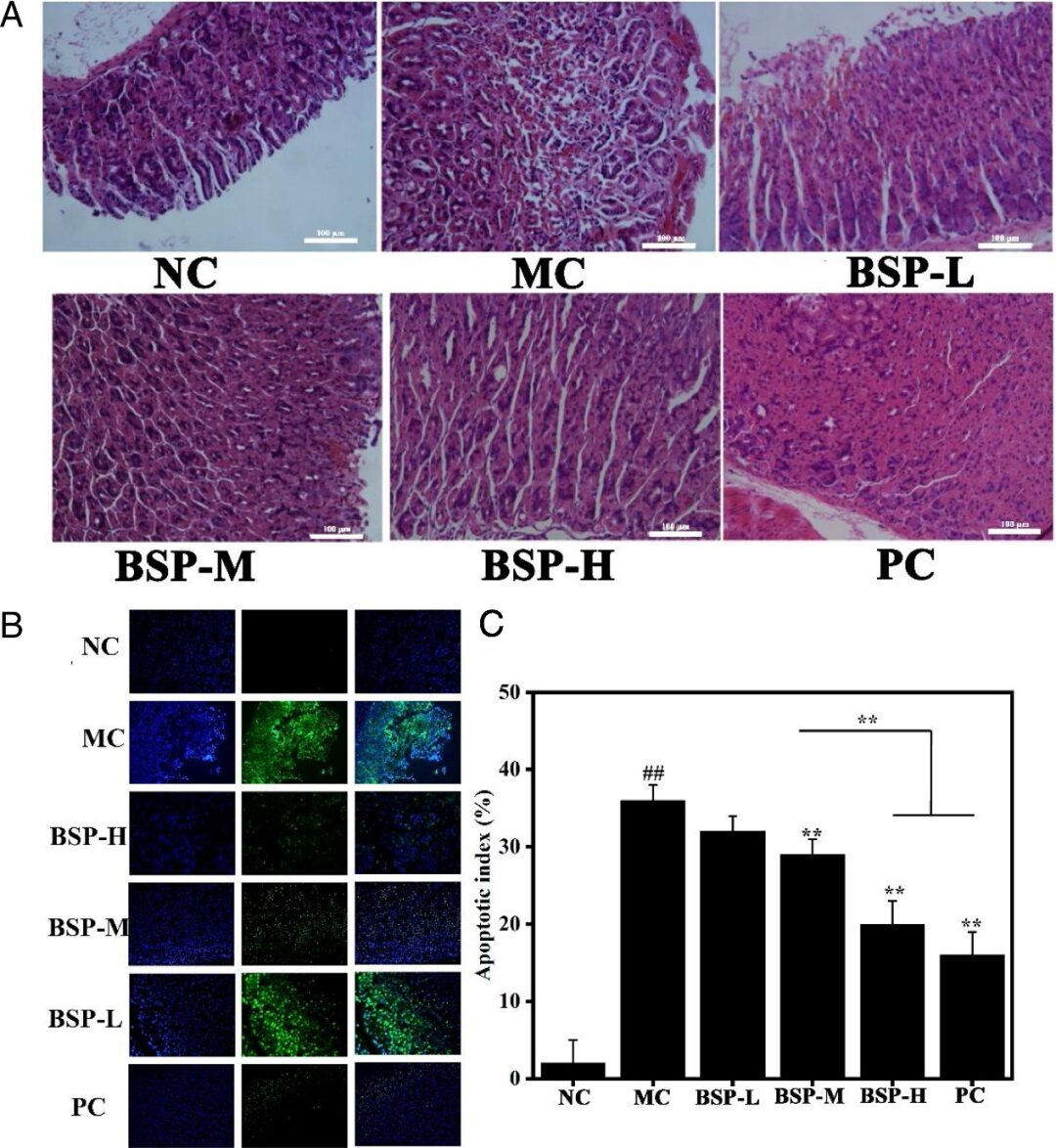
Figure 8: H&E (A), TUNEL (B) staining images and apoptosis index (C) of gastric tissues from different groups.
3
Summary and Reflection
This study extracted and purified the water-soluble polysaccharide BSP from Bletilla striata pseudobulbs and conducted preliminary characterization, finding that BSP consists of mannose and glucose (7.45:2.55), with a molecular weight of approximately 1.7×105 Da. Subsequently, the authors established in vitro and in vivo ethanol injury models to evaluate the gastroprotective effects of BSP, discovering that BSP exhibited significant protective effects against ethanol-induced gastric damage in both in vivo and in vitro settings. Furthermore, the authors further demonstrated that the gastroprotective mechanism of Bletilla striata polysaccharide may be related to its antioxidant activity, reflected in the increased activities of antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase) and reduced oxidative products (malondialdehyde, lipid peroxides). Additionally, Bletilla striata polysaccharide can also inhibit apoptosis of gastric tissue cells. Therefore, BSP may serve as a promising drug for preventing gastric injury. Numerous studies have indicated that Bletilla striata polysaccharide can also be used as a pharmaceutical excipient, and its excellent mucosal adhesion and gel properties can be explored for formulation research. In the future, Bletilla striata polysaccharide may reveal more possibilities in gastrointestinal therapy.
References:
[1] Ammar HH, Lajili S, Sakly N, et al. Influence of the uronic acid composition on the gastroprotective activity of alginates from three different genus of Tunisian brown algae. [J]. Food Chem, 2018, 239:165-171.
[2] Carlotto J, Maria-Ferreira D, de Souza LM, et al. A polysaccharide fraction from “ipê-roxo” (Handroanthus heptaphyllus) leaves with gastroprotective activity. [J]. Carbohydr Polym, 2019, 226:115239.
[3] Loboda A, Damulewicz M, Pyza E, et al. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. [J]. Cell Mol Life Sci, 2016, 73(17):3221-47.
[4] Monforte MT, Smeriglio A, Germanò MP, et al. Evaluation of antioxidant, anti-inflammatory, and gastroprotective properties of Rubus fruticosus L. [J]. Phytother Res, 2018, 32(7):1404-1414.
[5] Pawlaczyk-Graja I, Balicki S, Wilk KA. Effect of various extraction methods on the structure of polyphenolic-polysaccharide conjugates from Fragaria vesca L. leaf. [J]. Biol Macromol, 2019, 130:664-674.
[6] Peng Q, Li M, Xue F, Liu H. Structure and immunobiological activity of a new polysaccharide from Bletilla striata. [J]. Carbohydr Polym, 2014, 107:119-23.
Original link:https://doi.org/10.1016/j.carbpol.2020.116620
DOI: 10.1016/j.carbpol.2020.116620
Supervisor: Di Liuqing
Typesetting Editors: Zhang Xinru, Lu Yingying
Manuscript Reviewers: Wang Lingchong, Di Liuqing