Click the blue text to follow us! Abstract
Abstract
Macrophage phenotype dysregulation is a major cause of colitis, as it not only enhances oxidative stress and exacerbates inflammatory responses but is also closely related to dysbiosis of the gut microbiota. To effectively treat colitis, it is necessary to address these three issues simultaneously, but current treatments are unsatisfactory. Here, we developed a “three birds with one stone” probiotic, named Se@EcN-C2/A2, using Escherichia coli Nissle 1917 (EcN), a clinically approved probiotic, to intracellularly synthesize selenium (Se) nanoparticles through biomineralization. The surface of Se@EcN was coated with polyethylene glycol chitosan and sodium alginate, which endowed the probiotic with high resistance to harsh gastrointestinal environments and strong adhesion and targeting ability to inflamed areas of the colon, promoting the uptake of M1 macrophages. A2 metabolizes into SeCys2 and MetSeCys, participating in the synthesis of GPX 2 and TXNRD 1, leading to reactive oxygen species (ROS) clearance to inhibit Toll-like receptor and nuclear factor κB signaling pathways. In DSS-induced colitis mice, Se@EcN-C2/A2 exhibited satisfactory therapeutic and preventive effects, including the clearance of oxidative stress and modulation of macrophage phenotype to suppress inflammatory responses and restore intestinal barrier function. The viable probiotic EcN in the colon effectively regulated the dysbiosis of the microbiota by reducing the abundance of pathogenic E. coli and increasing the abundance of Lactobacillus and Bifidobacterium. This study, titled “Triple-Functional Probiotics with Intracellularly Synthesized Selenium Nanoparticles for Colitis Therapy by Regulating the Macrophage Phenotype and Modulating Gut Microbiota,” was published in ACS Nano.

 Background Introduction
Background Introduction
The etiology of colitis involves multiple factors, but recent studies have emphasized the critical role of dysregulated macrophage phenotypes and gut microbiota dysbiosis in the pathogenesis of colitis. Standard clinical treatments for colitis primarily include anti-inflammatory drugs (sulfasalazine and mesalamine) and immunosuppressive agents (steroids and anti-TNF-α antibodies). These drugs have achieved acceptable clinical effects in alleviating inflammatory symptoms; however, merely improving clinical symptoms often leads to relapse. Furthermore, a significant drawback of traditional treatments is the lack of specificity for the inflamed sites, which can lead to severe complications such as allergic reactions, hepatotoxicity, and even infections and malignancies. Therefore, there is an urgent need to develop effective drugs that target the inflamed sites and control the disease at its source, rather than just alleviating symptoms.
Macrophages are fundamental components of the innate immune system, acting as sentinels to clear pathogens and toxins to maintain intestinal homeostasis. However, dysregulated intestinal immune responses and damaged epithelial cells can lead to macrophage polarization towards a pro-inflammatory phenotype known as M1 macrophages. Single-cell RNA sequencing (scRNA-seq) has also revealed a significant increase in M1 macrophage populations in the colonic mucosa of patients with colitis. M1 macrophages produce large amounts of reactive oxygen species (ROS), which not only exacerbate epithelial cell damage but also induce activation of STAT-1, MAPK, and NF-κB, leading to an overall enhancement of the inflammatory response. The activation of the NF-κB signaling pathway in M1 macrophages induced by ROS leads to increased production of pro-inflammatory cytokines (such as TNF-α, IL-6, and IL-1β), resulting in a positive feedback loop that attracts more pro-inflammatory cells to the site of inflammation, thereby exacerbating the inflammatory state. However, these strategies do not address the root cause of colitis, leading to unsatisfactory therapeutic outcomes. Therefore, limiting the inflammatory response at its source by clearing ROS from M1 macrophages and inducing macrophages to polarize from the M1 phenotype to the anti-inflammatory M2 phenotype may be a promising strategy for treating colitis.
In addition to modulating macrophage polarization to suppress inflammatory signals in colitis patients, reshaping the dysregulated gut microbiota is also crucial for colitis treatment. Changes in the gut microbiota play a role in the pathogenesis of colitis. For example, dysbiosis leads to the secretion of pathogenic metabolites and pro-inflammatory cytokines by a large number of pathogens in the colon, thereby inducing and exacerbating colitis. Probiotics such as Bifidobacterium longum, Lactobacillus, and Escherichia coli Nissle 1917 (EcN) have been applied in clinical treatments for colitis to regulate gut microbiota, but unsatisfactory therapeutic effects have largely hindered their application, which may be related to the harsh gastrointestinal environment (low pH, digestive enzymes, and bile acids) that causes probiotics to lose viability and weaken their therapeutic effects, and the abnormal peristalsis of the inflamed colon hinders the colonization of probiotics. Recently, researchers have developed several strategies to modify probiotics for colitis treatment, enhancing their gastrointestinal resistance and intestinal colonization ability, such as biofilm/cell membrane coating and biointerface self-assembly/mineralization. However, probiotics alone cannot clear high levels of ROS and balance the excessively activated inflammatory response in the inflamed colon. Selenium (Se), as an essential trace nutrient for health, is incorporated into antioxidant selenoproteins such as GPX to regulate oxidative stress and maintain redox balance, thereby modulating physiological processes such as immunity, inflammation, and central nervous system function. Selenium deficiency can lead to increased accumulation of ROS and conversion of macrophages to the pro-inflammatory M1 phenotype; therefore, selenium can be used to improve the inflammatory response in colitis.
 Research Overview
Research Overview
Inspired by the clinical approval of the probiotic EcN for treating inflammatory gastrointestinal disorders, the authors’ team used EcN as a bioreactor to intracellularly synthesize Se nanoparticles (Scheme 1), which have two characteristics: regulating gut microbiota and reducing oxidative stress in the inflamed colon. The probiotics were coated with biocompatible polyethylene glycol chitosan and sodium alginate (Se@EcN-C2/A2) to enhance their tolerance to the harsh gastrointestinal environment, thereby improving their survival in the colon. At the inflamed sites, Se@EcN-C2/A2 can be effectively taken up by M1 macrophages, which have a higher uptake of Se@EcN-C2/A2. Se@EcN-C2/A2 is metabolized in M1 macrophages to SeCys2 and MetSeCys, forming antioxidant selenoproteins GPX 2 and TXNRD 1. Selenoproteins can effectively clear ROS from M1 macrophages, inhibit TLR and NF-κB signaling pathways, and activate the PI 3 K/AKT signaling pathway, leading to the polarization of M1 macrophages to M2 macrophages, resulting in decreased secretion of pro-inflammatory cytokines and increased secretion of anti-inflammatory cytokines. Additionally, the live probiotic Se@EcN-C2/A2 can also regulate the dysbiosis of gut microbiota in colitis mice by increasing the α-diversity of gut microbiota. Here, we developed a multifunctional probiotic to selectively target ROS at the site of inflammation, modulate gut microbiota, polarize macrophages, and balance the dysregulated gut microbiota, ultimately achieving satisfactory therapeutic effects in mouse colitis by controlling the disease at its source.
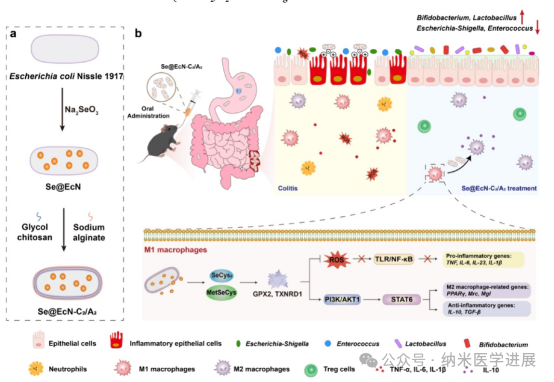
Figure 1. Schematic diagram of Se@EcN-C2/A2 improving DSS-induced colitis in mice. (a) Preparation of Se@EcN-C2/A2. (B) Se@EcN-C2/A2 improves DSS-induced colitis in mice by clearing ROS from M1 macrophages, inducing polarization of M1 macrophages to M2 macrophages, and improving gut microbiota. Se@EcN-C2/A2 is taken up by M1 macrophages and biotransformed into SeCys2 and MetSeCys to form antioxidant selenoproteins GPX 2 and TXNRD 1, which clear ROS, inhibit TLR and NF-κB pathways, and polarize macrophages from M1 phenotype to M2 phenotype by activating the PI 3 K/AKT signaling pathway.
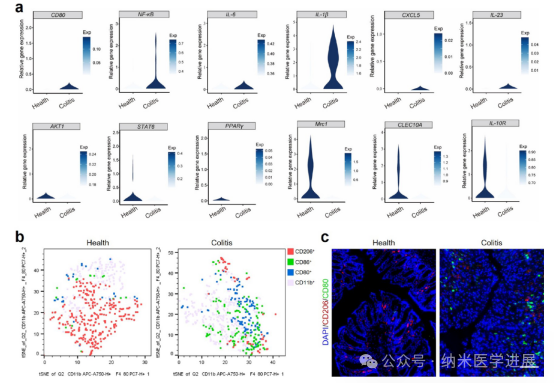
Figure 2. Phenotypes of macrophages in patients with colitis and DSS-induced colitis mice. (a) Violin plots showing changes in the expression of macrophage-related genes (M1 macrophages: CD 80, NF-κB, IL-6, IL-1β, CXCL 5, and IL-23; M2 macrophages: AKT1, STAT6, PPARγ, Mrc1, CLEC10A, and IL-10R). (http://scibd.cn/). (B) t-SNE plots showing different macrophage clusters in healthy and colitis mice colons. (c) Immunofluorescence staining of CD 80 and CD 206 in healthy and colitis mice colons (scale bar: 100 μm).
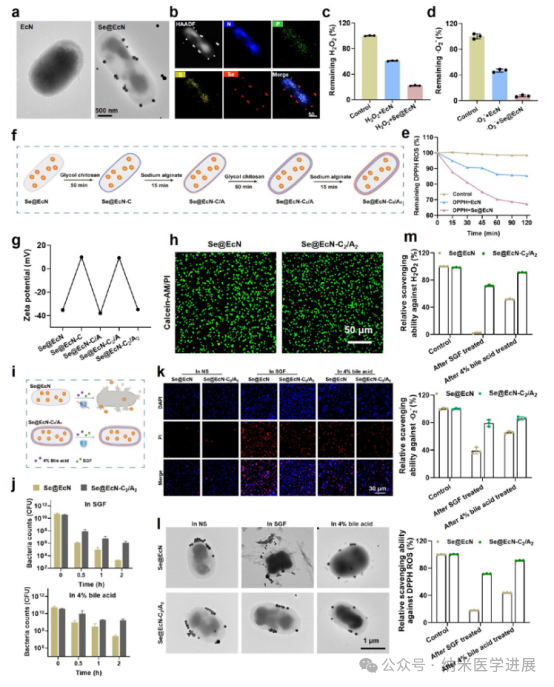
Figure 3. Preparation and characterization of Se@EcN-C2/A2. (a) TEM images of EcN and Se@EcN. (B) Se@EcN. H2O2 (c) ·O2− (d), (e) Clearance properties of Se@EcN. (f) Schematic diagram of the preparation of Se@EcN-C2/A2 by a layer-by-layer strategy. (g) Zeta potential of the bacteria during the coating with polyethylene glycol chitosan and sodium alginate used to visualize Se@EcN-C2/A2. (h) Live (green) and dead (red) staining of bacteria detected by FV 3000 confocal laser scanning microscopy (CLSM) after coating with polyethylene glycol chitosan and sodium alginate. (j) Survival quantification of Se@EcN and Se@EcN-C2/A2 exposed to SGF and 4% sodium alginate. (k) Dead bacterial staining (red) of Se@EcN and Se@EcN-C2/A2 after exposure to SGF or 4% bile acids for 2 hours. (l) Morphology of Se@EcN and Se@EcN-C2/A2 after exposure to SGF or 4% bile acids for 2 hours. Bile acids acted for 2 h, and Se@EcN and Se@EcN-C2/A2 were collected to detect the clearance ability of Se@EcN and Se@EcN-C2/A2 against H2O2−, ·O2−, and DPPH-ROS (n = 3).
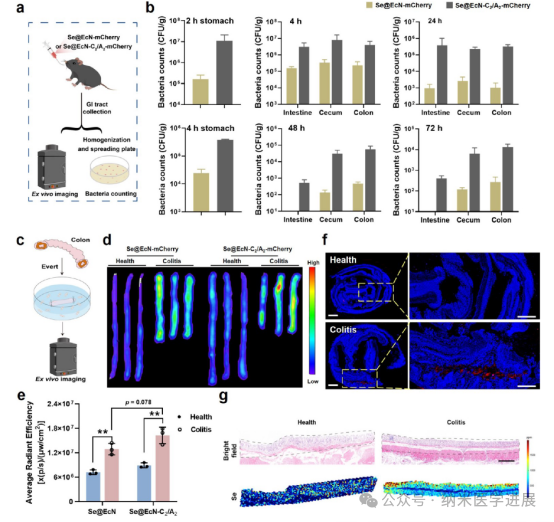
Figure 4. Gastrointestinal adhesion and targeting ability of Se@EcN-C2/A2. (a) Schematic diagram used to verify the enhanced survival, adhesion, and colonization of Se@EcN-C2/A2 in the gastrointestinal tract. (B) Number of bacteria surviving in the contents and tissues of the stomach, intestine, cecum, and colon after oral administration of Se@EcN-mCherry or Se@EcN-C2/A2-mCherry at 2, 4, 24, and 48 hours. (c) Schematic diagram of healthy colon (referred to as healthy) or colitis (d) in vitro fluorescence images, and (e) semi-quantitative fluorescence signal analysis of healthy colon or colitis colon after incubation with Se@EcN-mCherry or Se@EcN-C2/A2-mCherry for 6 hours. (f) Bacterial fluorescence staining of healthy colon or colitis colon after treatment with Se@EcN-C2/A2-mCherry for 6 hours (blue fluorescence signal: nucleus; red fluorescence signal: (g) Distribution of Se in healthy colon or colitis colon after treatment with Se@EcN-C2/A2-mCherry detected by LA-ICP-MS. The black dashed line shows the distribution of Se detected in colon tissues.
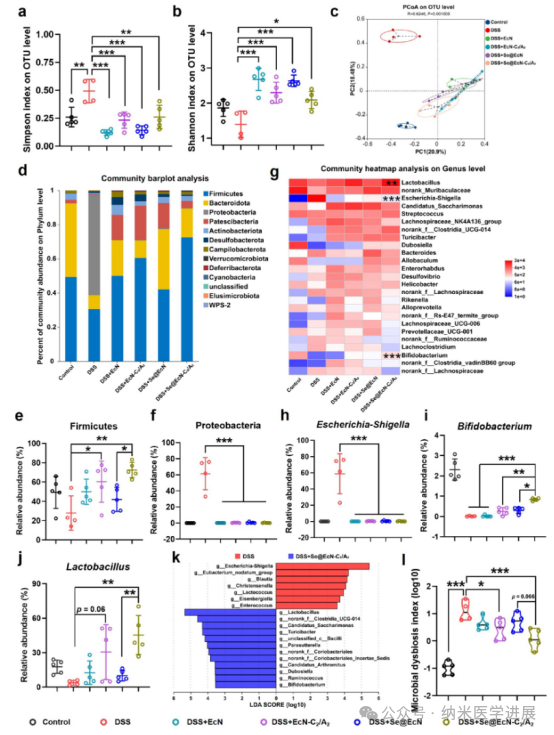
Figure 5. Effects of Se@EcN-C2/A2 on antioxidant, anti-inflammatory, and reprogramming macrophage phenotypes at the cellular level. Detection of intracellular ROS levels (a), DCF-positive cell ratio (B), and MFI of DCF (c) histogram (n = 3) in RAW 264.7 cells after different treatments by flow cytometry. (d) Fluorescence signal of DHE detected in RAW 264.7 cells after different treatments by FV 3000 CLSM. Flow cytometry detected the expression levels of TNF-α, iNOS, IL-6, and CD 206 in RAW 264.7 cells after different treatments, and qPCR detected the expression of IL-10 in RAW 264.7 cells after different treatments (n = 3). (k) Schematic diagram of the antioxidant, anti-inflammatory, and reprogramming macrophage phenotype effects of Se@EcN-C2/A2.
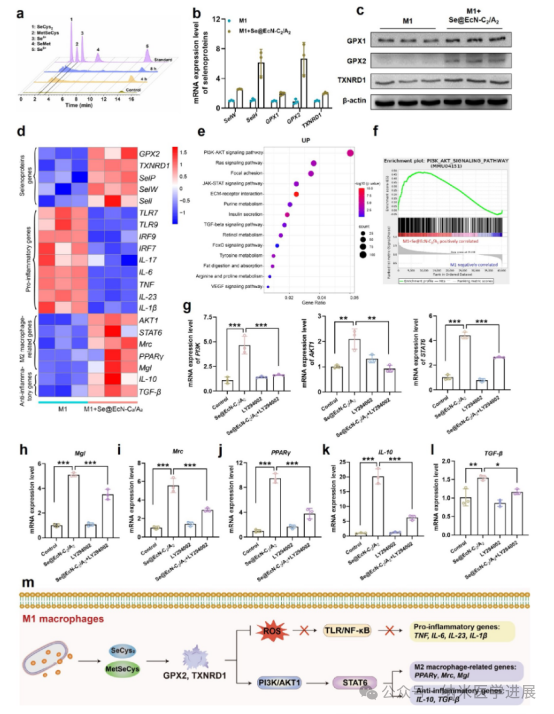
Figure 6. Mechanism by which Se@EcN-C2/A2 alleviates the inflammatory response of M1 macrophages. (a) Detection of Se-related metabolites in M1 macrophages after incubation with Se@EcN-C2/A2 by HPLC-ICP-MS. (B) mRNA levels of certain selenoproteins in M1 macrophages after incubation with Se@EcN-C2/A2 detected by qPCR (n = 3). (c) WB analysis detected the expression of selenoproteins in M1 macrophages after incubation with Se@EcN-C2/A2 (n = 3). Transcriptional analysis of M1 macrophages after incubation with Se@EcN-C2/A2. (d) Selenoprotein-related, pro-inflammatory-related, macrophage polarization-related, (e) KEGG pathway enrichment analysis of upregulated genes in the M1 + Se@EcN-C2/A2 group compared to the M1 group. GSEA enrichment plots show a positive correlation of the PI 3 K/AKT signaling pathway with the M1 + Se@EcN-C2/A2 group. mRNA levels of PI 3 K, AKT 1, and STAT 6 in M1 macrophages after administration of LY 294002 (h-1) Mgl (h), Mrc (i), PPARγ (j), IL-10 (k), IL-10 (k), IL-10 (k), IL-10 (k) and IL-10 (k). (m) Schematic diagram of the mechanism by which Se@EcN-C2/A2 weakens the inflammatory response in M1 macrophages and induces macrophage polarization.
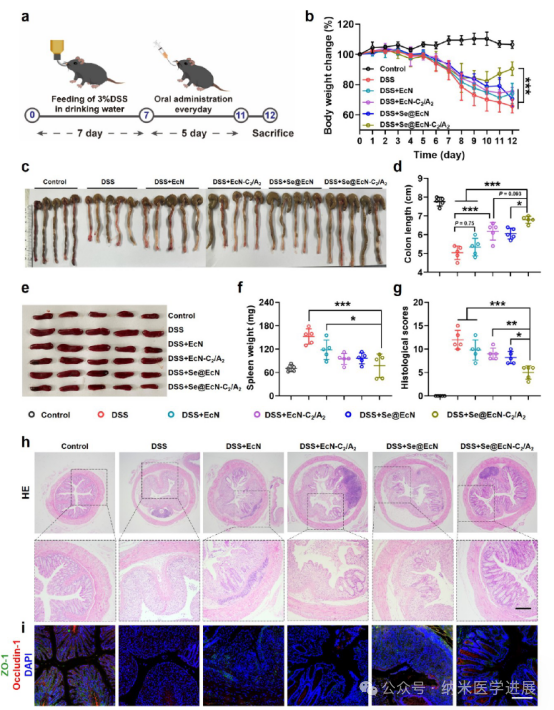
Figure 7. Therapeutic effects of Se@EcN-C2/A2 on DSS-induced colitis in mice. (a) Schematic diagram of the treatment program of Se@EcN-C2/A2 on DSS-induced colitis in mice. (B) Daily changes in body weight of mice in each group during treatment (n = 5). (c, d) Colon photographs (c) and colon lengths (d) of different groups on day 12 (n = 5). (e, f) Spleen photographs and spleen weights of different groups on day 12 (n = 5). (g, h) HE staining and histological scores of colon tissues after different treatments on day 12 (n = 5). (i) Immunofluorescence staining analysis of ZO-1 and Occludin-1 in colon tissues after different treatments on day 12. Scale bar: 200 μm.
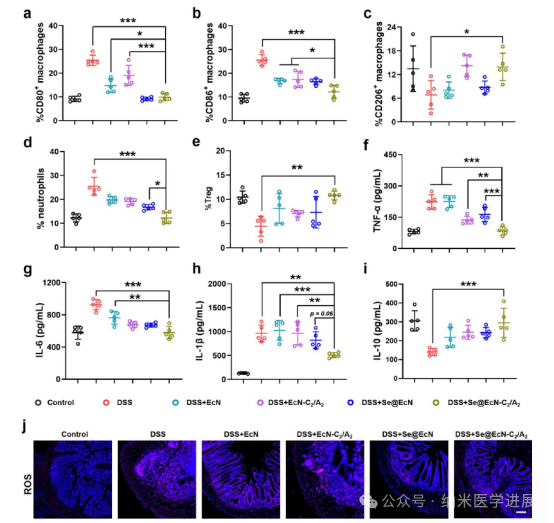
Figure 8. Se@EcN-C2/A2 reprogramming of macrophages and antioxidant effects in DSS-induced colitis mice. (a-e) Proportions of CD 80+ macrophages (a), CD 86+ macrophages (B), CD 206+ macrophages (c), CD 11 B + Ly 6 C + Ly 6 G+ neutrophils (d), and CD 3 + CD 4 + CD 25 + FoxP 3 + Treg cells in the colon of different groups (e) (n = 5). (f-i) Secretion of pro-inflammatory cytokines TNF-α (f), IL-6 (g), IL-1β (h), and secretion of anti-inflammatory cytokine IL-10 (i) in the colon of different groups (n = 5). (j) Immunofluorescence staining of ROS in colon tissues of different treatments. Scale bar: 200 μm.

Figure 9. Regulation of gut microbiota by Se@EcN-C2/A2 in DSS-induced colitis mice. Alpha diversity indices of Simpson index (a) and Shannon index (B) in different groups. (c) PCoA plots showing β-diversity of gut microbiota in different groups. (d) Bar graphs showing the relative abundance of gut microbiota at the phylum level in different groups. (e, f) Relative abundance of Firmicutes (e) and Bacteroidetes (f). (g) Heatmap of the relative abundance of the top 25 microbial genera. (h-j) Abundance of Lactobacillus (h), Bifidobacterium (i), and Lactococcus (j). Abundance is expressed as a relative percentage. (k) LEfSe analysis identified characteristic bacteria at the genus level in different groups (1) Dysbiosis index of gut microbiota in different groups (control group, DSS + EcN group, DSS + EcN-C2/A2 group, DSS + Se@EcN group, and DSS + Se@EcN-C2/A2 group, n = 5; DSS group, n = 4).
 Conclusion and Discussion
Conclusion and Discussion
This study utilized EcN as a bioreactor to intracellularly synthesize Se nanoparticles through biomineralization, resulting in Se@EcN, which was then coated with highly biocompatible polyethylene glycol chitosan and sodium alginate to obtain Se@EcN-C2/A2. A2 can resist gastrointestinal attacks and specifically adhere to and target inflamed sites to promote the uptake of M1 macrophages. Se@EcN-C2/A2 is metabolized in M1 macrophages to SeCys2 and MetSeCys, participating in the formation of antioxidant selenoproteins GPX 2 and TXNRD 1. Subsequently, the antioxidant selenoproteins can effectively clear intracellular reactive oxygen species, inhibit TLR and NF-κB signaling pathways, and activate the PI 3 K/AKT signaling pathway, leading to the polarization of macrophage phenotypes from M1 to M2. When EcN reaches the colon, the coating endows EcN with activity; then, the probiotic EcN effectively regulates the dysregulated gut microbiota to increase α-diversity and optimize microbial composition. Importantly, Se@EcN-C2/A2 exhibits high biosafety and has good therapeutic and preventive effects on DSS-induced colitis in mice, providing new insights for clinical treatment of related diseases.
Paper link (DOI):
https://doi.org/10.1021/acsnano.5c00574
Disclaimer: This WeChat account forwards content solely for academic exchange purposes and does not serve commercial purposes, nor does it represent support or endorsement of its views. If there are issues related to intellectual property protection or other matters, please contact the editor in a timely manner, and we will coordinate to address them as soon as possible. The copyright of original articles from this WeChat account belongs to this WeChat account, and sharing on non-media platforms such as Moments is welcome.
Contact the editor for free submissions. This public account also has a real-name academic exchange group for nanomedicine. Please note your name + institution + research direction to add the WeChat number below.
Previous recommendations: Zhang Xuesong from the General Hospital of the People’s Liberation Army published in Advanced Materials: Microenvironment Adaptive Nanomedicine Promotes Spinal Cord Repair by Inhibiting Inflammatory Cascade Responses and Neuronal Apoptosis
Click “Read the original text” to access the original paper
「ENDING」


Submission WeChat|nanomedicine1
Submission Email|[email protected]
Follow Nanomedicine Progress
Learn about the Frontiers of Nanomedicine
Long press the QR code to submit privately