

[Research Background]
In traditional metal oxide cathode materials during charge and discharge processes, the insertion/extraction of alkali metal (lithium, sodium, potassium, etc.) ions requires charge compensation provided by transition metals, meaning that oxidation-reduction reactions occur as electrons are gained and lost. The number of electrons that can be transferred is one of the main limiting factors for the charge-discharge specific capacity of cathode materials. In recent years, with a deeper understanding of the charge-discharge processes and reaction mechanisms of materials, it has been found that the anionic (mostly oxygen) oxidation-reduction reactions (ARR), which were previously sought to be avoided, can occur reversibly and provide higher energy to cathode materials beyond traditional mechanisms. However, materials relying on this mechanism often also exhibit significant voltage hysteresis, low initial Coulomb efficiency, and poor mechanical performance, which reduces the energy storage efficiency and power output density of the battery, thus limiting their practical application. These issues are often regarded as intrinsic characteristics of ARR, although the exact relationship between them has not yet been clarified.
Na2/3Ni1/3Mn2/3O2 is a classic sodium-ion battery cathode material that exhibits almost no voltage hysteresis, high initial efficiency, and excellent kinetic performance in specific charge-discharge ranges. In this material, Ni is in the +2 oxidation state and can be oxidized to +4, which exactly compensates for the charge transfer of 2/3 of Na extraction. Coupled with the highly reversible charge-discharge behavior of this material, it has long been believed that only Ni oxidation-reduction reactions participate in charge compensation during the charge-discharge process. Recently, some studies have suggested that there may be signs of oxygen participating in charge compensation, while other studies oppose this view. Clearly, clarifying whether ARR provides charge compensation based on this material’s highly reversible charge-discharge behavior is crucial for a deeper understanding of the relationship between adverse factors such as voltage hysteresis and ARR.
[Work Introduction]
Recently, Dai Kehua from Tianjin Normal University, Liu Gao from Lawrence Berkeley National Laboratory, Yang Wanli, and others meticulously prepared ex-situ samples of Na2/3Ni1/3Mn2/3O2 (hereinafter referred to as NNMO) under different charge-discharge states. They analyzed the chemical state changes of oxygen during the charge-discharge process of NNMO using the unique super high detection efficiency full-field resonant inelastic X-ray scattering (mRIXS) technique developed at the Berkeley Laboratory ALS. By combining the analysis results of X-ray absorption spectroscopy (XAS) for cation electronic states, it was found that the anionic oxidation-reduction shows a strong and reversible signal in the electrochemical cycling of NNMO.
The article was published in the internationally renowned journal Nano Energy. Dai Kehua is the first author, and Dai Kehua, Liu Gao, and Yang Wanli are the corresponding authors.
[Content Description]
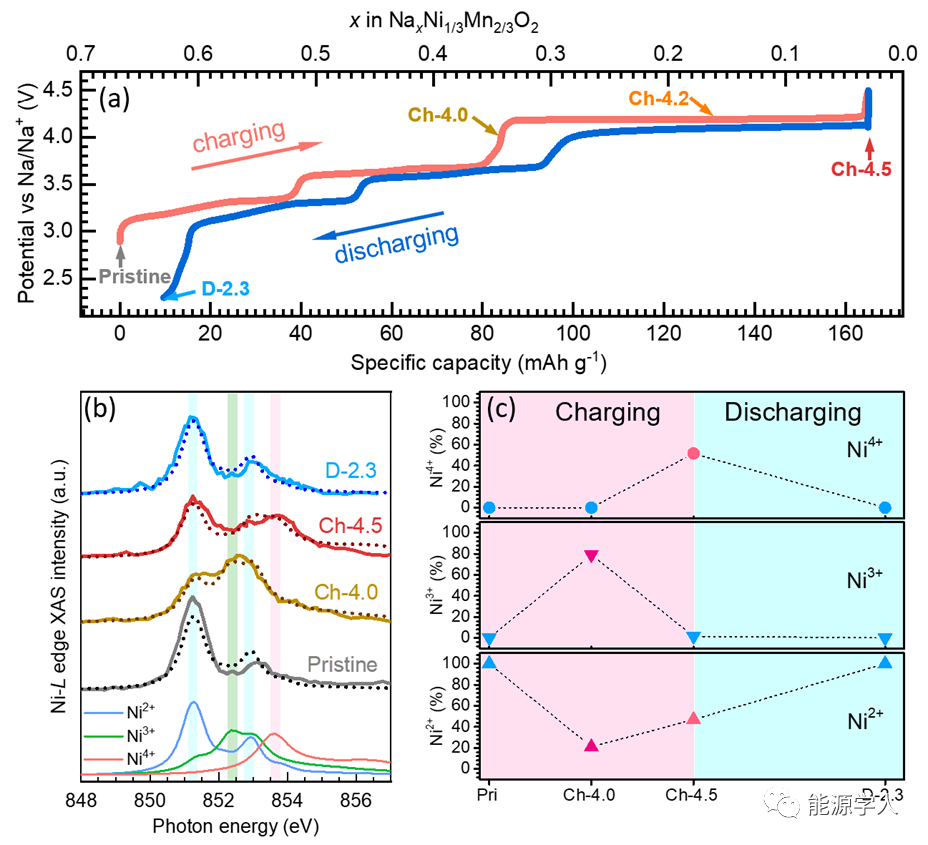
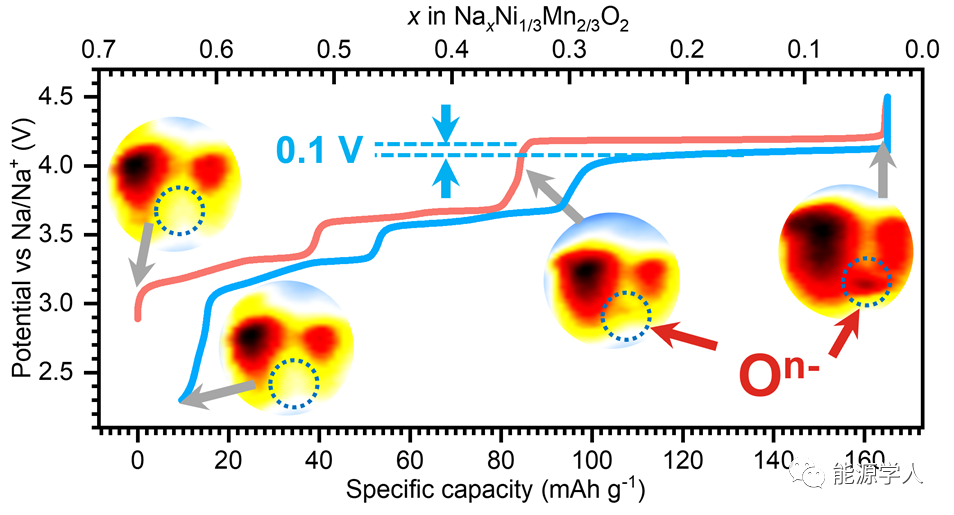
Figure 1. Initial charge-discharge curves and the evolution of Ni and Mn valence states. (a) Charge-discharge curve of NNMO at a 0.1C rate. (b) X-ray absorption spectrum of Ni-L3 edge in TFY mode (solid line) and the fitted curve (dashed line). (c) Evolution of Ni valence states at different charge-discharge states.
From the highly reversible initial charge-discharge curve in Figure 1a, it can be seen that NNMO has extremely low voltage hysteresis (the maximum voltage difference between charge and discharge platforms does not exceed 0.1 V) and high initial Coulomb efficiency. Figure 1b shows the X-ray absorption spectrum of Ni-L3 edge in TFY mode and the spectrum obtained by linearly overlapping standard spectra of different valence states of Ni. Through this linear fitting, the Ni valence state distribution at different SOCs as shown in Figure 1c can be obtained. The original material Ni appears as fully +2 valence. From pristine to charging up to 4.0 V, 80% of Ni2+ transforms into Ni3+. After fully charging to 4.5V, Ni3+ almost completely disappears. However, surprisingly, both Ni2+ and Ni4+ increase simultaneously, indicating that this process involves the reduction/oxidation/disproportionation reaction of Ni3+. The reduction of Ni3+ corresponds to the reductive coupling mechanism of transition metal-oxygen at high voltage. More importantly, quantitative data reveal that during the charge and discharge processes, Ni oxidation-reduction only compensates for 0.35 mol of Na+ extraction, indicating that there must be other charge compensation mechanisms present.
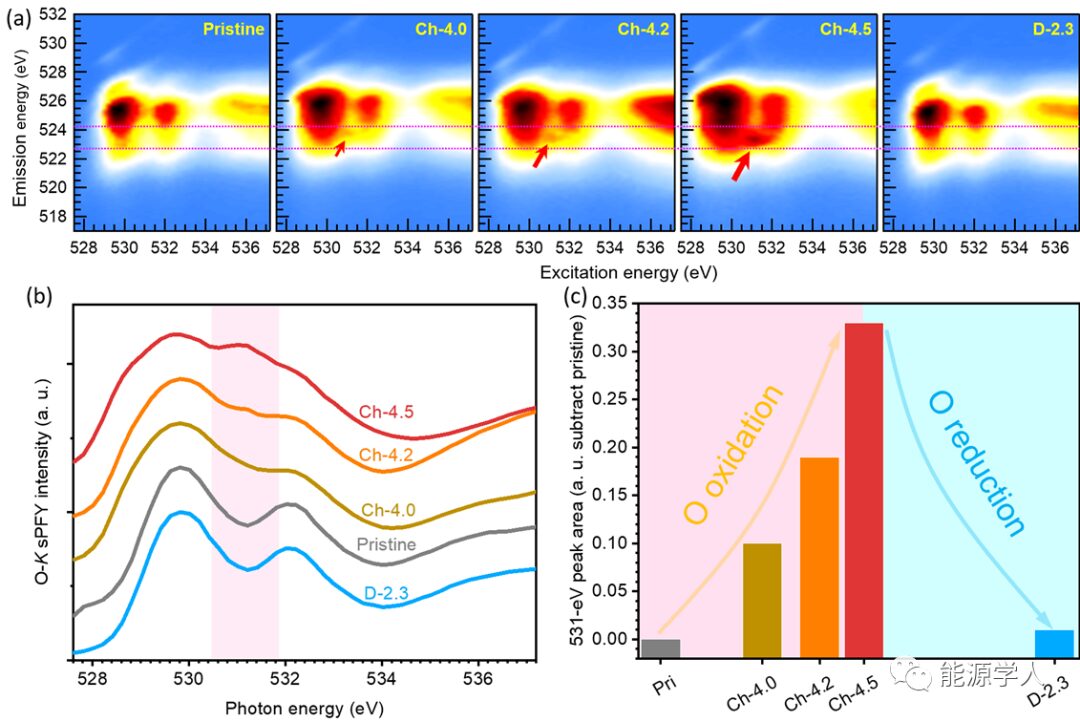
Figure 2. Chemical behavior of oxygen during the first cycle of NNMO. (a) O-K mRIXS images at different SOCs. The ARR characteristic peak is indicated by the red arrow. (b) sPFY spectrum obtained from partial integration between the two horizontal dashed lines in (a). (c) Area of the mRIXS-sPFY 531 eV peak (pink shaded area).
Considering that Mn maintains a +4 valence throughout the initial charge-discharge process (see Supporting Info of the original text), the only element that may provide more charge compensation is oxygen. Therefore, Figure 2 demonstrates the changes of oxygen during the first cycle. Figure 2a displays the O-K mRIXS images at different SOCs, showing that a weak ARR (On-) characteristic peak appears at Ch-4.0, gradually intensifying as charging progresses, then disappearing at the fully discharged state. Figure 2b is the sPFY spectrum obtained from partial integration between the two horizontal dashed lines in Figure 2a, and Figure 2c shows the integral area around 531 eV in the pink shaded region. For detailed analysis of this derived spectrum and the relationship with ARR, please refer to Joule 3 (2), 518-541. Figure 2c clearly indicates that there exists highly reversible ARR during the charge and discharge of NNMO.
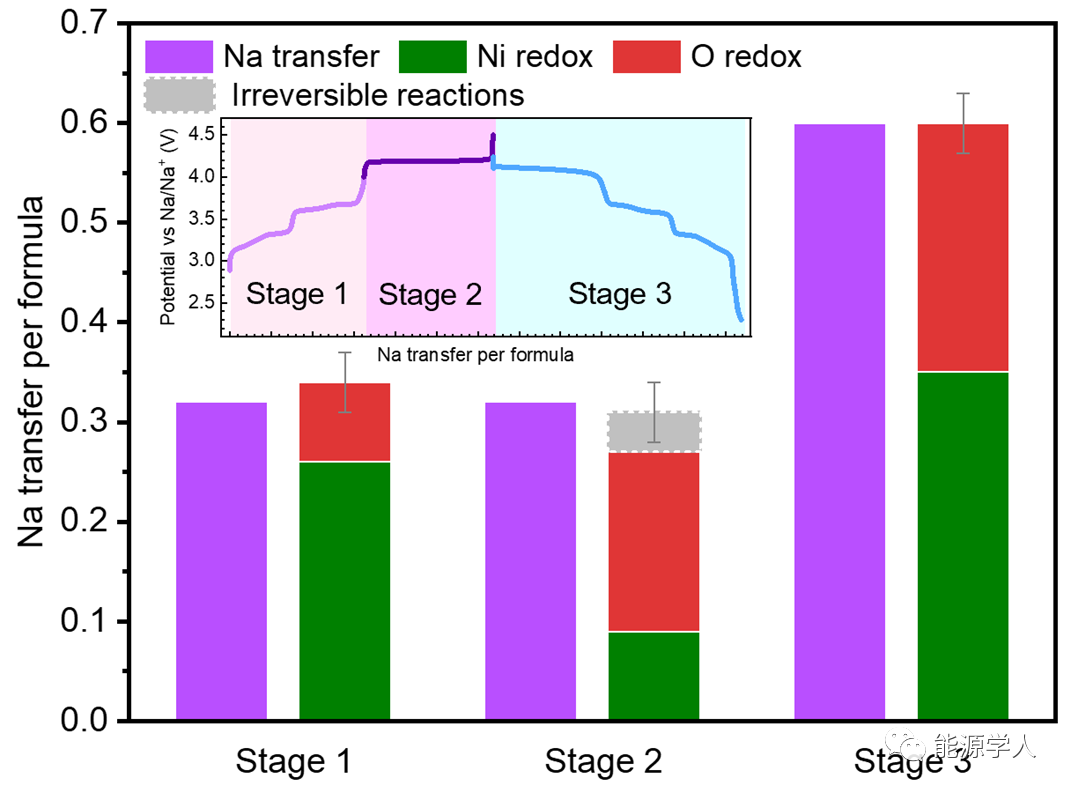
Figure 3. Quantitative analysis of the oxidation-reduction reactions present in NNMO charge-discharge.
Combining the previous spectral analysis of Ni and O reactions and the electrochemical capacity representing the amount of Na extraction/insertion, a quantitative analysis of the oxidation-reduction reactions present in NNMO charge-discharge can be conducted, with the final results shown in Figure 3, where the red part represents the charge compensation provided by ARR, which evidently plays an important role in this system.
[Conclusion]
This study clearly demonstrates that reversible ARR plays an important role in the charge-discharge process of Na2/3Ni1/3Mn2/3O2, particularly in the 4.2 V charge-discharge plateau. This finding is surprising, as this material is composed solely of third-period transition metal elements, and its voltage hysteresis is small enough to be negligible, while the electrochemical curves are highly reversible. This article confirms that highly reversible (up to 96%) ARR can also exist in traditional materials with high voltage and highly reversible charge-discharge curves.
At the same time, these research results raise several questions worth further investigation. First, NNMO is a system where only 3d TMs exist in the traditional TM-O layer. This fundamentally differs from other Na=2/3 compounds containing Li or Mg in the TM layer (which have strong ARR), as the highly ionic bonding between Li/Mg and O is similar to lithium-rich systems. Strangely, as a non-lithium-rich system, NNMO does not “need” ARR to compensate for charge transfer during cycling, because Ni oxidation-reduction is already “sufficient”. In other words, the results of this article indicate that the traditional understanding that anionic oxidation only occurs after transition metals are fully oxidized is evidently not comprehensive enough. How to explain this phenomenon warrants further research.
Secondly, during the charging process of NNMO, the oxidation-reduction reactions of cations and anions occur simultaneously. It is currently unclear whether this simultaneous reaction of cations and anions helps stabilize ARR and/or improve its kinetics; however, this behavior sharply contrasts with most other ARR systems, including lithium-rich manganese-based and Na2/3Mg1/3Mn2/3O2 materials, where TM oxidation-reduction and ARR occur sequentially. In-depth study of this unique phenomenon of simultaneous cation-anion oxidation-reduction in NNMO is also an important topic for the future.
In summary, the discovery of ARR in NNMO suggests that it is feasible to enhance the capacity of certain traditional 3d-TM materials by introducing ARR, and more importantly, it denies the inevitable connection between ARR and some performance issues. Further experimental and theoretical studies on Na2/3Ni1/3Mn2/3O2 series materials will help further optimize high-capacity materials containing ARR.
Kehua Dai, Jing Mao, Zengqing Zhuo, Yan Feng, Wenfeng Mao, Guo Ai, Feng Pan, Yi-de Chuang, Gao Liu, Wanli Yang, Negligible voltage hysteresis with strong anionic redox in conventional battery electrode, Nano Energy, 2020, 104831, DOI:10.1016/j.nanoen.2020.104831
Author Introduction:
Dai Kehua, currently an associate professor at the College of Chemistry, Tianjin Normal University. He studied at the School of Chemistry and Molecular Engineering, Peking University from 1998 to 2008, obtaining a bachelor’s and a doctoral degree in science. From 2008 to 2019, he worked at the College of Metallurgy, Northeastern University. He served as a visiting researcher and senior visiting researcher at Lawrence Berkeley National Laboratory from 2013 to 2014 and from 2016 to 2018, respectively. In 2019, he was selected as a distinguished professor and young scholar in Tianjin, and officially joined the College of Chemistry, Tianjin Normal University in January 2020. He has published more than ten papers as the first author/corresponding author in high-level international journals such as Joule, Nano Energy, ACS Appl. Mater. Interface, J. Power Sources. He proposed and achieved a quantitative characterization method for lattice oxygen oxidation-reduction in lithium/sodium-ion battery cathode materials, confirming that highly reversible oxygen oxidation-reduction can exist in third-period transition metal oxide materials (Joule 2019, 3, 518-541). He elucidated the relationship between the generation of surface Mn2+ during the charge-discharge process of Na0.44MnO2 and capacity decay (Nano Energy 2015, 16, 186-195). He proposed the PVP (polyvinylpyrrolidone) gel combustion method, which utilizes the amide groups on the PVP chain to fix metal ions, achieving better mixing than other polymers due to its chelation effect, and enabling rapid combustion to obtain monodisperse particles, thus preparing crystalline products with uniform composition and no impurities. By optimizing the high-temperature reaction process, he successfully overcame issues such as excessive sintering, surface defects, and decomposition at high temperatures, preparing a variety of high-performance micron-scale single-crystal lithium-sodium-ion battery cathode materials.
Liu Gao, currently a tenured researcher at Lawrence Berkeley National Laboratory, leads a cross-PI applied energy materials research team and oversees several research programs for the U.S. Department of Energy. He graduated from the Department of Technical Physics at Peking University with a bachelor’s degree in science; he obtained his doctoral degree in science from Michigan State University. His research areas include lithium-ion and sodium-ion battery cathode and anode materials and conductive binders, lithium-sulfur batteries, all-solid-state batteries, and biomimetic materials. He has received multiple awards, including the U.S. Department of Energy R&D 100 Award. He has published numerous papers in journals such as Joule, Advanced Materials, JACS, Nature Communications, and Nano Energy, with total citations exceeding 14,000.
Yang Wanli: currently a tenured researcher at Lawrence Berkeley National Laboratory. His main research directions include materials physics, soft X-ray spectroscopy methodology, soft X-ray spectroscopy instrument development, and energy materials science. He established a precise quantitative method for the oxidation state of metals through soft X-ray spectroscopy. He led the construction of the world’s first ultra-high-efficiency soft X-ray resonant inelastic scattering device focused on energy materials research and introduced its application into the field of energy materials research, achieving reliable testing of oxygen oxidation-reduction (O redox) in battery electrodes, clarifying many misunderstandings about soft X-ray spectroscopy in the field, and continually advancing experimental techniques, analytical methods, and related theories of soft X-ray spectroscopy in energy materials research.