
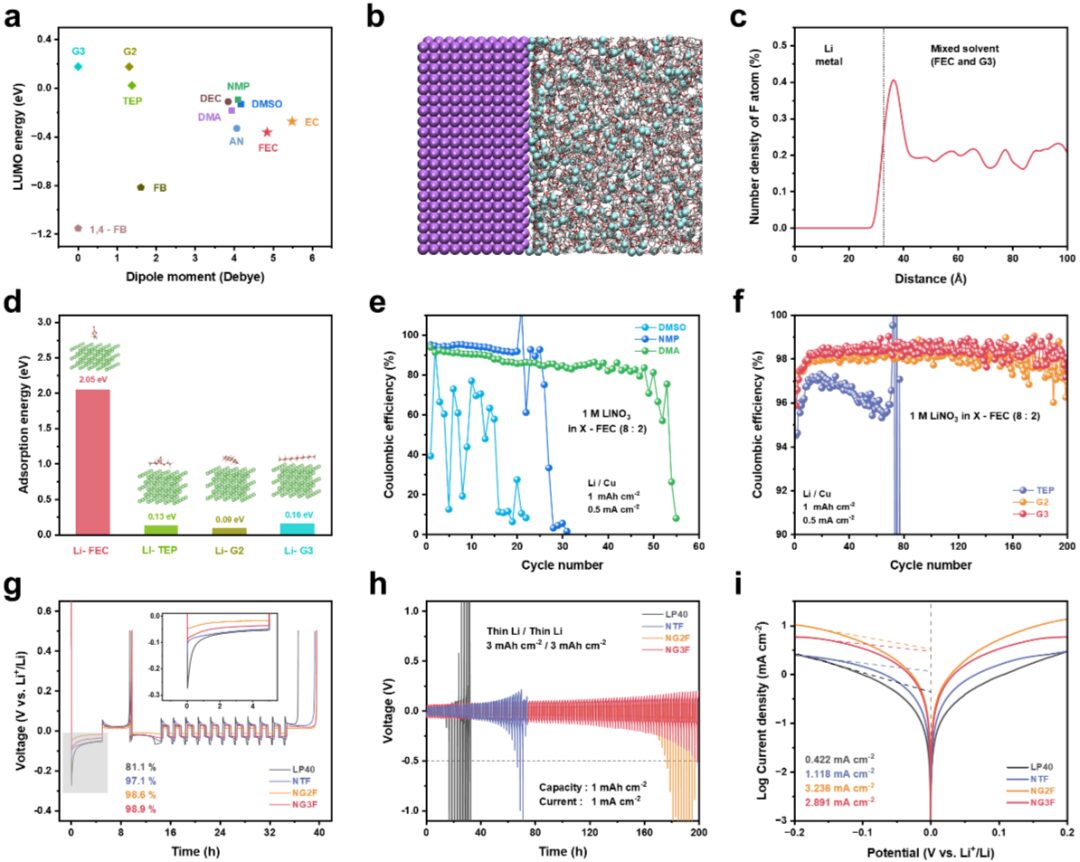
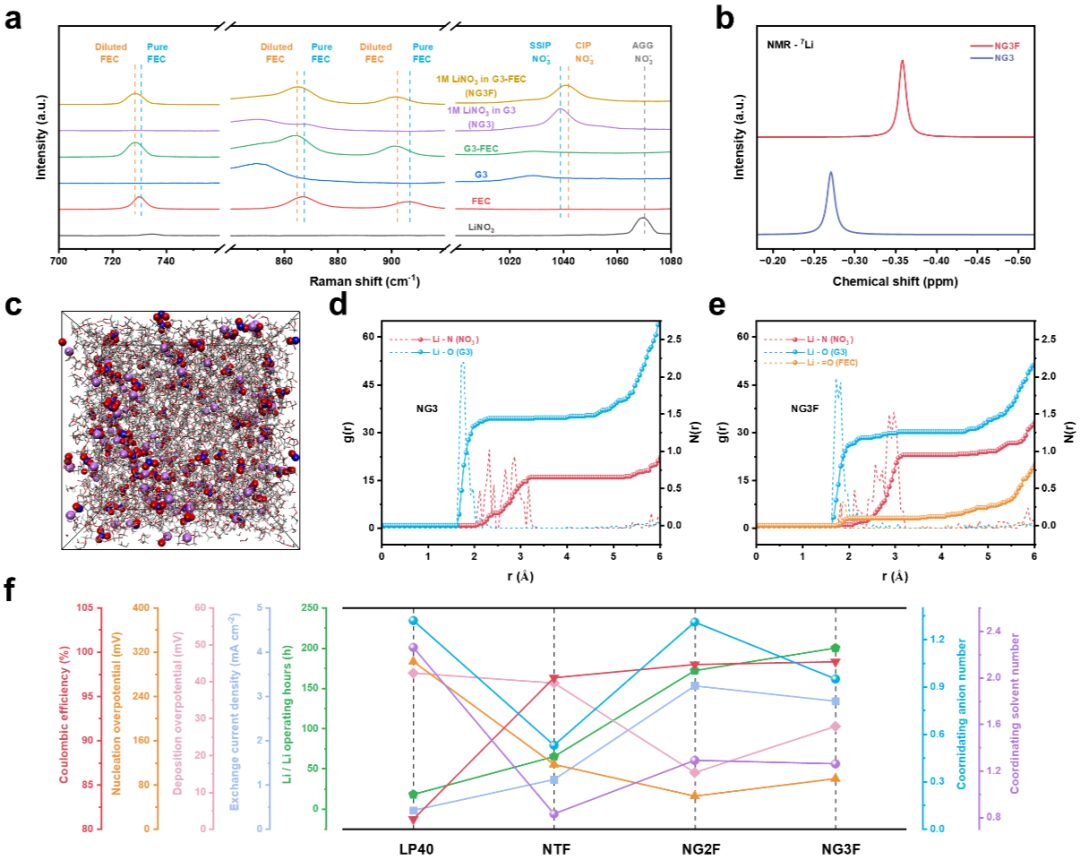
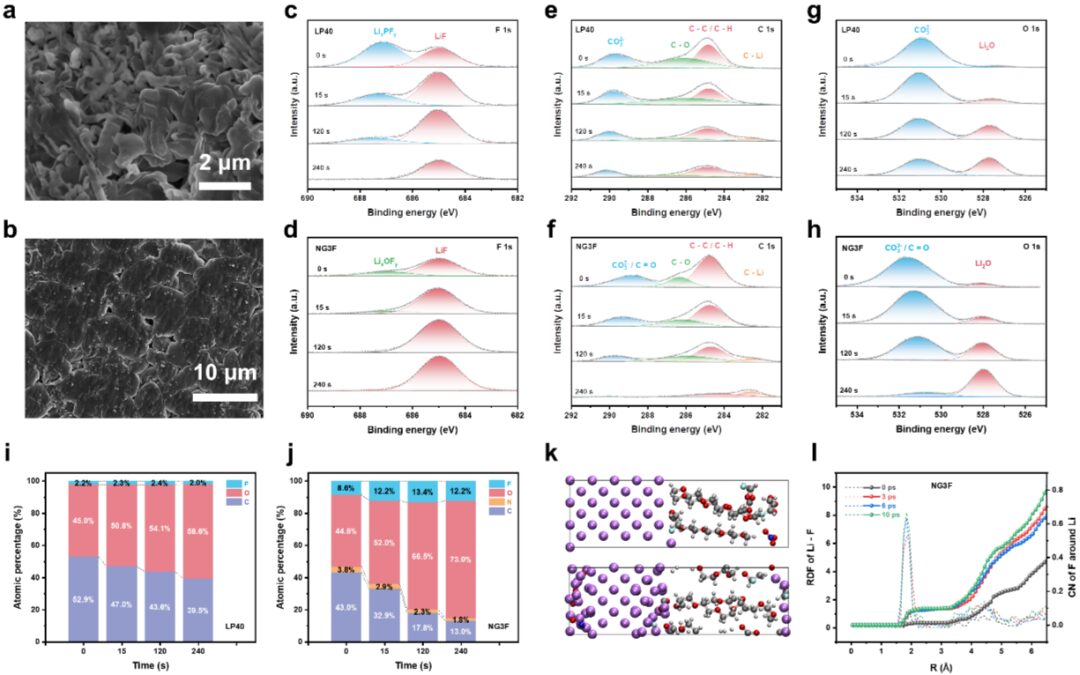
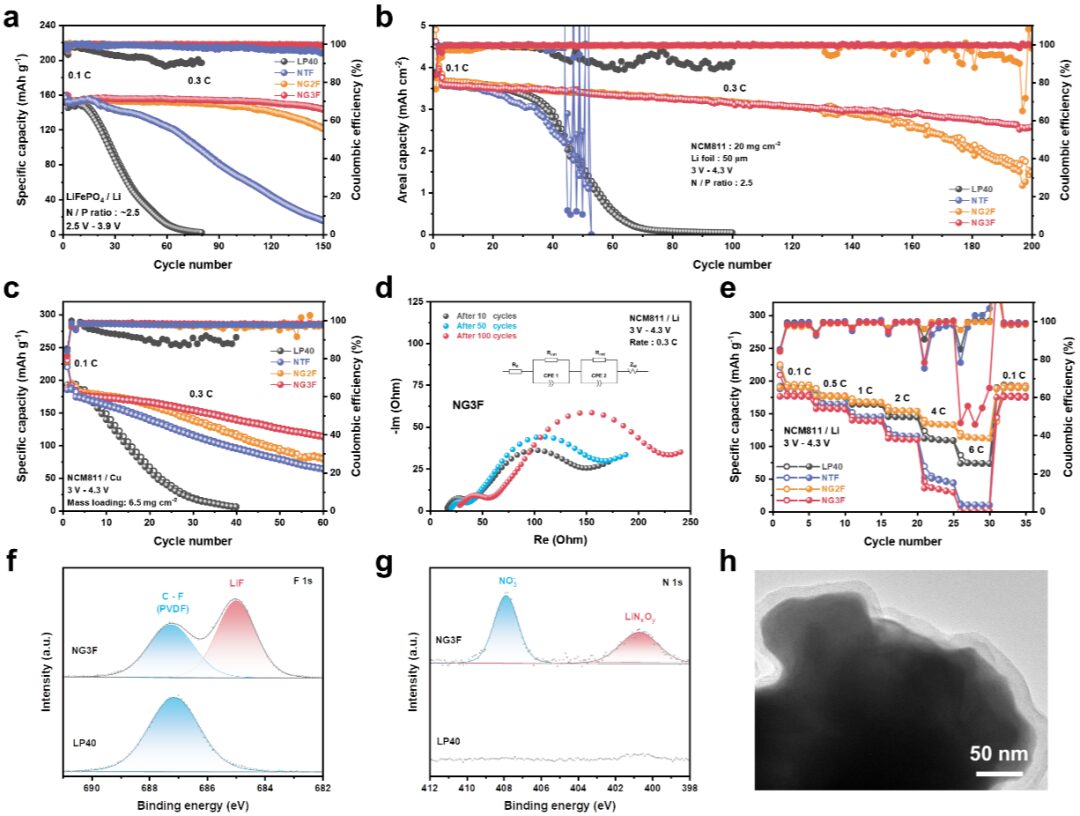
Negative Electrode-Free Alkali Metal Batteries: From Laboratory to Practical Applications
2024-05-27

In-Situ Liquid Phase Synthesis of High Activity Material Content Composite Cathodes to Boost High-Performance All-Solid-State Lithium Batteries
2024-05-27

Academician Zhao Tianshou from Southern University of Science and Technology AEM: Strategies for Constructing Ultra-High Volume Ratio Capacity Ge Anodes
2024-05-27

Professor Ren Xiangzhong’s team at Shenzhen University AEM: Glutamic Acid Induced Proton Substitution of Sodium Vanadate Cathodes Promotes High Performance in Aqueous Zinc Ion Batteries
2024-05-27

Beijing University of Technology Bai Ying/Wu Chuan/Zhao Ran EnSM: Multi-Level Regulation of pH and Interface Environment to Aid Highly Reversible Zinc Anodes!
2024-05-26

Front. Energy | “Artificial Photosynthesis Towards Carbon Neutrality” Special Issue
2024-05-26

Nanjing University Zuo Jinglin/Jin Zhong/Ma Jing/Yuan Shuai Matter: Construction of Novel Redox Active MOFs and Their Application in Lithium-Sulfur Battery Research
2024-05-26

Interface Polarization Induction Effects of Ultra-Thin Dielectric Nanosheets Achieve High-Performance Solid-State Lithium Metal Batteries
2024-05-26
