Cavities (tooth decay) are an unresolved public health issue affecting over 2.3 billion people worldwide, particularly among the impoverished and those with poor medical conditions. Notably, iron deficiency anemia is closely related to severe cavities. Current preventive measures are insufficient for at-risk populations, especially in severe cases where high-sugar diets and poor oral hygiene lead to rapid accumulation of pathogenic biofilms, resulting in cavities that are difficult to control effectively. In the biofilm that induces caries (caries-causing), microorganisms form highly organized and protective bio-structures, creating localized acidic pH microenvironments that promote the growth of cariogenic bacteria and the acid dissolution of enamel. Existing antimicrobial approaches are limited to broad-spectrum drugs, which lack efficacy and target specificity against cariogenic biofilms, resulting in limited effectiveness against cavities. To overcome these challenges, new anti-biofilm strategies are needed to target the acidogenic properties found under high-caries risk conditions (sugar exposure and poor oral hygiene) in at-risk populations.
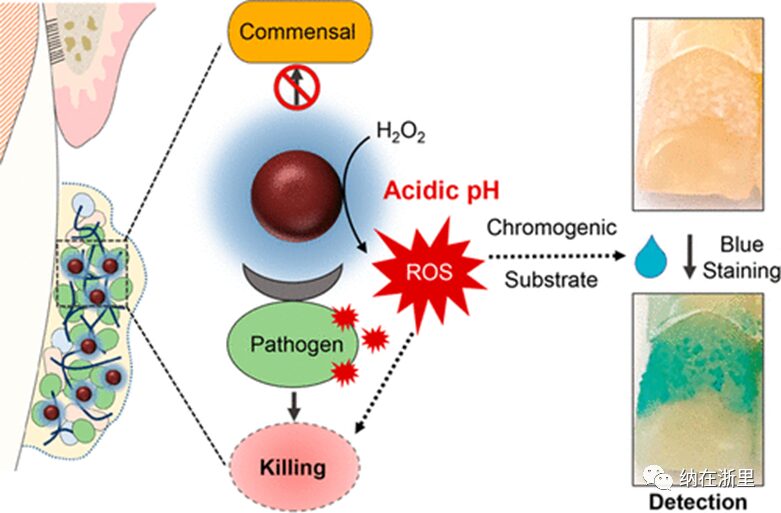
Bioactive nanoparticles have shown significant multifunctionality against microorganisms in biofilms, from enhanced permeation and drug delivery to on-demand activation in response to environmental stimuli. Among these, catalytic iron oxide nanoparticles (also known as nanozymes) have rapidly emerged due to their good therapeutic activity in animal models. A formulation of iron oxide nanoparticles known as FerIONP has been reported and approved by the U.S. Food and Drug Administration (FDA) for the treatment of iron deficiency anemia. When used topically in rodent models, it can activate hydrogen peroxide through catalytic (peroxidase-like) activity to disrupt cariogenic biofilms. However, its biological effects on biofilms formed in the human mouth are not yet clear, which is critical for assessing the targeted efficacy and clinical translatability of FerIONP-based approaches.
Research Methods
1.FerIONP as an Anti-Biofilm and Cavities Prevention Therapy
To mimic high-risk cavity conditions in susceptible populations (high sugar intake and poor oral hygiene), we designed an intraoral model (Figure 1B) that allows natural biofilms to form on enamel surfaces under frequent sucrose exposure without mechanical biofilm removal (brushing). In this model, treatments can be directly applied to biofilm-covered enamel specimens, allowing the entire intact specimen to be restored with minimal disturbance (Figure 1C), enabling simultaneous assessment of local (in situ) effects on biofilm accumulation and enamel demineralization. Additionally, specimens were exposed to sugar (20% sucrose, V/V) solution four times a day without mechanical cleaning (brushing) to simulate high-cavity risk conditions (Figure 1C). The authors analyzed and compared the bioactivity of the local application of the FerIONP/H2O2 group (twice daily; after breakfast and before bed) with the blank control and the use of /H2O2 alone. The topical dose of FerIONP (1.5 mg daily) was based on previous studies and was 340 times less than the FDA-approved systemic dose (intravenous). The low amount of FerIONP used here effectively catalyzed H2O2 at acidic pH through peroxidase-like activity (Figure 1A). Subjects wore custom appliances during the three treatment periods and kept the specimens in situ (Figure 1D).
At the end of each study period, the intraoral devices were removed, and the biofilms were analyzed for microbiology (live cells) and confocal imaging (spatial organization). The authors found no impact on the total viable cell count in the biofilms treated with FerIONP/H2O2 (Figure 2A). Previous studies indicated strong killing effects against carcinogenic substances in vitro, particularly against S. mutans, suggesting selective antimicrobial activity. To explore this possibility, the researchers analyzed a subset of data from subjects with baseline detection of S. mutans. Notably, it was observed that the mutants were completely eradicated in the biofilms treated with FerIONP/H2O2 (Figure 2B). Confocal images also confirmed the microbiological data, showing that the biofilms containing S. mutans cells (green) were effectively suppressed after treatment with FerIONP/H2O2 (Figure 2C), indicating targeting against this organism.
Next, the researchers evaluated the effect of FerIONP/H2O2 on enamel demineralization (decay) using surface microhardness (SMH) analysis. Despite daily sucrose exposure inducing a high-cavity challenge, only FerIONP/H2O2 showed significantly reduced enamel demineralization compared to the blank control group (Figure 2D). This therapeutic effect was more pronounced in individuals carrying S. mutans (Figure 2E). This is particularly noteworthy since individuals with S. mutans showed higher levels of enamel demineralization. Overall, these data demonstrate the anti-biofilm and anti-cavity properties of FerIONP/H2O2 under severe cavity conditions in humans. Additionally, FerIONP/H2O2 showed more targeted therapeutic effects in subjects carrying S. mutans, a common cariogenic pathogen in severe cavity cases.
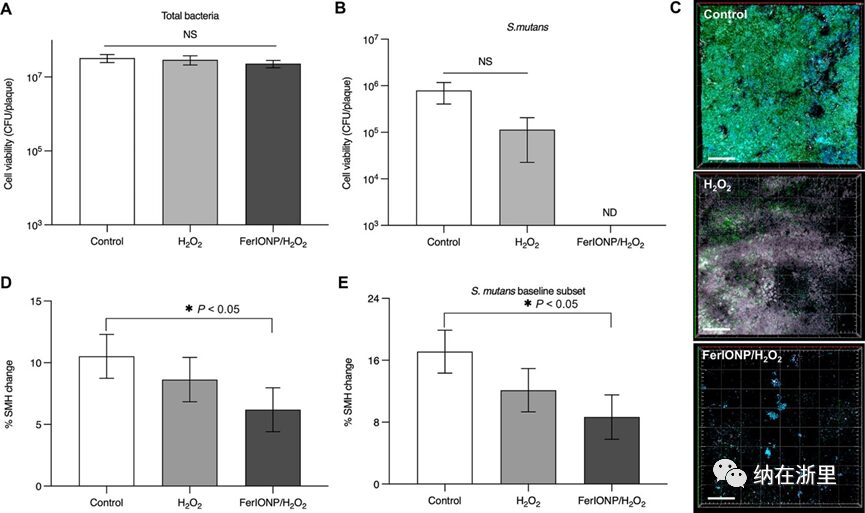
Figure 2 Biofilm and Enamel Analysis
2.FerIONP Catalytic H2O2 Action Combines with Mutants and Preferentially Kills Mutants
Given the potential targeting action of FerIONP/H2O2, the authors hypothesized:
(1) FerIONP can preferentially bind to mutants compared to other species;
(2) Thus, generating ROS in close proximity to kill this organism more effectively at acidic pH.
To investigate these issues, FerIONP (1 mg/mL) was incubated with S. mutans or other oral commensal streptococci (including S. oralis, S. gordonii, and S. sanguinis) and assessed for binding. As shown in Figure 3A, FerIONP showed significantly more binding to S. mutans compared to other strains, indicating higher binding affinity for S. mutans. After incubation with FerIONP, cell viability was further assessed by adding H2O2. The data showed that enhanced binding led to more effective killing of mutants upon exposure to H2O2 (with a reduction in viable counts of >4 logs compared to control), while other species were not significantly affected.
The differences in FerIONP binding and subsequent killing may be due to interactions between FerIONP and the surface proteins of the mutants. FerIONP is a nanoparticle composed of an iron oxide core coated with carboxymethyl dextran, which can specifically bind to mutants as this organism expresses several cell membrane-associated glucan-binding proteins. In addition to glucosyltransferases (Gtfs, particularly GtfB and GtfC), at least two glucan-binding proteins (Gbps; GbpA and GbpC) are uniquely expressed at high levels by the mutants, which can directly mediate binding interactions with glucan or glucan-coated surfaces. To evaluate this hypothesis, mutants with Gbp and Gtf mutations were used to study the binding capacity of FerIONP. It was found that the bacterial binding capacity to FerIONP significantly decreased, with much lower iron binding amounts in Gbp or Gtf mutant strains compared to wild-type (Figure 3C). Interestingly, the absence of GbpA or GbpC significantly weakened the binding of FerIONP to the mutants. GbpA is a secreted protein associated with the cell surface, while GbpC appears to be anchored in the cell wall. GbpA has a high proportion of β-sheet in its carboxyl-terminal region, which may provide optimal folding and facilitate glucan binding.
In contrast, GbpC has structural similarities to the antigen I/II V region, containing unique circular regions in similar lectin-like β-epithelial and interstitial folds that allow for high-affinity binding to specific glucans. Therefore, the glucan-binding interactions mediated by GbpA and GbpC may be different, yet equally effective. Whether the absence of GbpC affects the synthesis and secretion of GbpA protein or the binding affinity for glucans, and vice versa, may provide further insights into the binding mechanism of FerIONP. Nevertheless, our findings may partially explain the enhanced killing activity of FerIONP/H2O2 against pathogens, as its preferential binding to mutants leads to localized generation of ROS near bacterial cells, especially considering the short lifespan of ROS, which does not diffuse over long distances.
To further elucidate the selective killing of bacteria, high-resolution fluorescence imaging techniques were employed to simultaneously analyze cell viability and ROS production in situ. To observe the distribution of live and dead bacteria, SYTO 60 was used to label intact bacteria, while propidium iodide (PI) was used to determine non-viable cells. For ROS, the fluorescent probe, hydroxyphenyl fluorescein (HPF), was used to detect hydroxyl radicals. FerIONP (1 mg/mL) was added to actively growing bacterial cells (S. mutans or S. oralis) and exposed to H2O2, followed by observation using confocal microscopy. It was found that after treatment with FerIONP/H2O2, most S. mutans cells were killed (red), accompanied by a significant amount of ROS (purple) produced in situ (Figure 3D). In contrast, only a small number of S. oralis cells were affected, and the observed ROS was much lower. Interestingly, the spatial distribution of ROS signals matched the location of dead cells, indicating that bacterial death primarily resulted from oxidative damage. Consistent with our confocal findings, quantitative analysis confirmed that most S. mutans cells were killed compared to S. oralis (Figure 3E), with substantial ROS production (Figure 3F).
These findings indicate that FerIONP exhibits a dual-action mode.
(1) FerIONP has higher specificity in binding to mutants compared to other species,
(2) This enables the catalytic action of H2O2 to generate antimicrobial radicals on-site (directly on the bacterial surface). This mechanism is crucial for targeting specificity, as FerIONP’s binding affinity to commensal streptococci (which can produce H2O2) is poor, thus protecting them from self-harm.
Therefore, a key concept is achieved through the peroxidase-like activity of FerIONP to realize “selective binding and enhanced killing of mutants,” targeting pathogens in the presence of H2O2 without harming commensals.
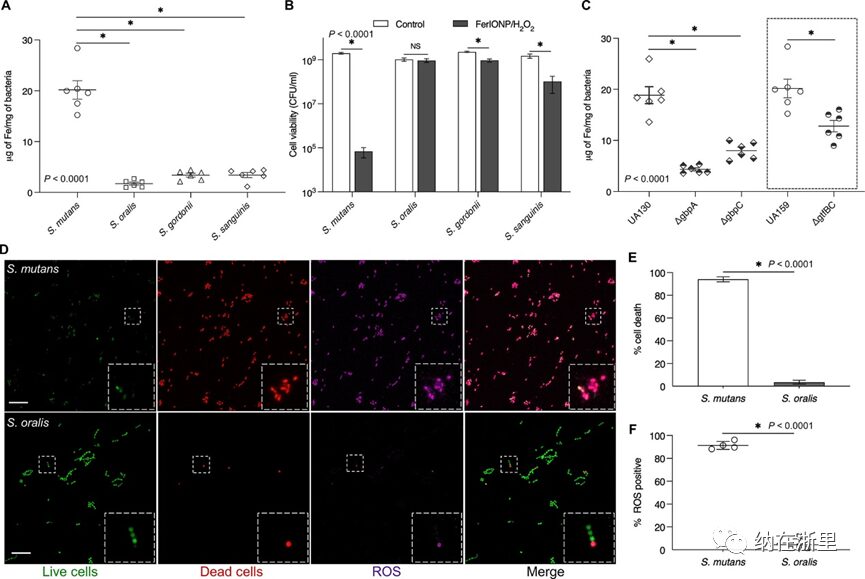
Figure 3 FerIONP Preferential Binding and Killing of Pathogens
3.FerIONP Detects Pathogenic Biofilms
Given the preferential binding of FerIONP to mutants and the catalytic action of H2O2 at acidic pH, FerIONP can be utilized for the detection of cariogenic biofilms. To test this possibility, the authors constructed a biofilm model using S. mutans and S. oralis grown on saliva-coated hydroxyapatite discs as single or mixed-species biofilms. For the mixed-species system, microorganisms were grown under high-sugar or low-sugar conditions, producing acidic or non-acidic biofilms, respectively. The single species and mixed species biofilms were treated with FerIONP (1 mg/mL) and washed to remove unbound material, followed by immediate colorimetric detection using 3,3′,5,5′-tetramethylbenzidine (TMB). FerIONP can oxidize the colorless TMB (via free radicals generated by H2O2) to a blue product, which can be quantified by measuring absorbance at 652 nm. It was found that mutant biofilms exhibited a prominent blue stain, with significantly higher absorbance than biofilms from S. oralis (Figure 4A). This observation was further confirmed in mixed-species biofilms grown under high-sugar (acidic) conditions, enriched with mutants (compared to the high proportion of S. oralis under low-sugar conditions, Figure 4B). Using a human tooth model (Figure 4C), similar blue staining was observed in biofilms of S. mutans formed on natural teeth under cariogenic conditions (high sugar and acidic pH), indicating the diagnostic potential of FerIONP.
Previous investigations have shown that catalytic iron oxide nanoparticles (also known as nanozymes) exhibit antibacterial and anti-biofilm properties in vitro and in animal models. However, nanozymes have not been tested in humans, which could further elucidate their bioactivity and targeting characteristics. Here, the authors demonstrate that FerIONP disrupts pathogenic biofilms through selective binding activation mechanisms at low doses and inhibits enamel demineralization under severe conditions in the human mouth. Importantly, no adverse symptoms in the oral cavity or systemic reactions were observed during the treatment. These findings could promote its clinical translation for cavity prevention, especially important given that current approaches are insufficient for susceptible populations unable to effectively control cariogenic biofilms.
One direct application of FerIONP is related to the association between iron deficiency anemia and aggressive cavities. Common risk factors associated with severe childhood cavities and iron deficiency include high dietary sugar intake, malnutrition, and poor oral hygiene. High sucrose consumption and poor oral care promote the accumulation of typically acidogenic biofilms rich in S. mutans. The preferential binding of FerIONP to S. mutans and its catalytic activation at acidic pH are advantageous under severe cavity conditions without affecting commensals, providing a more targeted approach. Given that FerIONP has been used in children and adults, it offers the possibility of adopting these FDA-approved nanoparticles tailored for cavity prevention in high-risk patients with iron deficiency anemia.
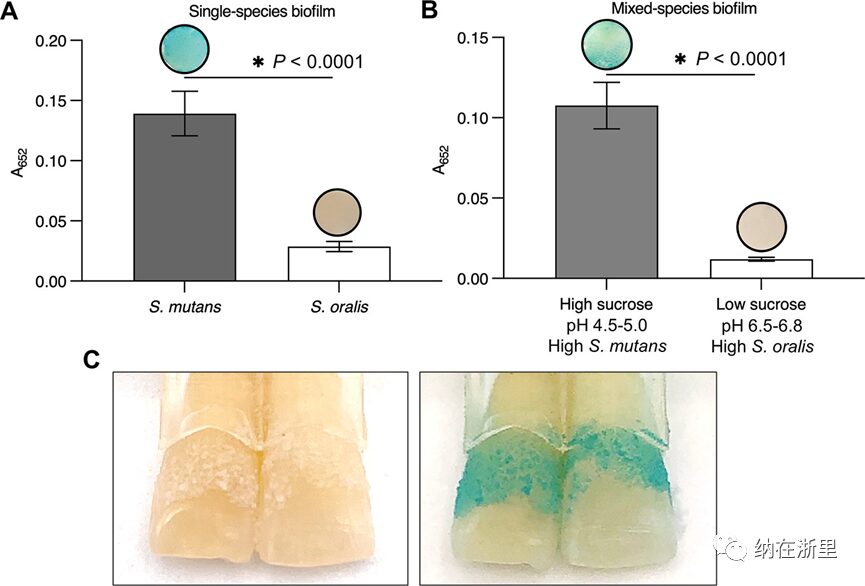
Figure 4 FerIONP Detects Pathogenic Biofilms
Conclusion
This study conducted FerIONP treatment on human subjects under conditions conducive to cavities using wearable intraoral devices implanted with natural enamel. It was found that FerIONP displayed powerful antimicrobial specificity against biofilms containing S. mutans (a cariogenic pathogen) but not against other oral bacteria, leading to a significant reduction in enamel demineralization. Further analysis showed that FerIONP preferentially binds to S. mutans through a glucan-binding mechanism and selectively kills pathogens by generating reactive oxygen species (ROS) in situ. Additionally, the potential of utilizing catalytic mechanisms to detect cariogenic biofilms with FerIONP was demonstrated. The first study in humans is proposed, proving the potential therapeutic application of catalytic iron oxide nanoparticles (nanozymes) as targeted nanomedicines against oral infectious diseases.
References:Liu Y, Huang Y, Kim D, et al. Ferumoxytol nanoparticles target biofilms causing tooth decay in the human mouth[J]. Nano Letters, 2021, 21(22): 9442-9449.



