
Bone defects are a common and challenging issue in the medical field. The development of biomaterials with excellent bone repair capabilities and cost-effectiveness is of significant practical importance for the clinical treatment of bone defects. In recent years, tissue-engineered scaffolds have become increasingly popular for the treatment of bone defects. Notably, the process of bone repair is not only influenced by the composition, mechanical properties, and structural characteristics of the scaffold but is also directly affected by the biological signals released from the scaffold. The process of bone repair is extremely complex, involving inflammatory, repair, and remodeling phases, each requiring a coordinated regulation of a series of bioactive molecules. Therefore, an ideal scaffold should be able to control the release of different drugs according to the bone tissue healing process. Specifically, antibacterial drugs can be released early after implantation to prevent bacterial infection, while drugs with angiogenic and osteogenic activities can be released slowly in the later stages to ensure angiogenesis and osteogenesis.3D printing technology is currently one of the most widely used and effective methods for constructing bone tissue engineering scaffolds. It allows for the customization of the scaffold’s shape, size, and pore structure to meet the requirements for cell adhesion, diffusion, and the subsequent processes of angiogenesis and osteogenesis necessary for bone repair. Poly(L-lactic acid) (PLLA) is a biodegradable polymer that has been widely used in the manufacture of bone tissue engineering scaffolds through 3D printing in recent years. However, PLLA scaffolds have certain limitations for bone repair, such as poor mechanical properties and limited cell affinity. Additionally, as a bone repair material, PLLA scaffolds may be affected by bacterial infection and inflammation in the early stages after implantation, and later face challenges in attracting relevant cell proliferation and differentiation to promote osteogenesis and angiogenesis. To mitigate early bacterial infections in implants, the most common clinical approach is the use of antibiotics. However, the widespread use of antibiotics can lead to bacterial resistance, resulting in an urgent need for novel and effective antibacterial agents. Plant-derived natural antibacterial agents have garnered significant interest due to their ability to effectively disrupt microbial cells without causing resistance.

Professor Luo Binghong’s team at Jinan University utilized layered double hydroxides (LDHs), a two-dimensional inorganic nanomaterial, to efficiently load osteogenic and angiogenic dimethyl oxalylglycine (DMOG) based on anion exchange. Furthermore, the LDHs loaded with DMOG and the natural antibacterial agent eugenol were simultaneously modified onto the surface of 3D printed poly(L-lactic acid) (PLLA) scaffolds through polydopamine layers, thereby constructing a 3D printed scaffold capable of achieving spatiotemporal controlled release of various bioactive drugs. Specifically, eugenol is rapidly released in the early stages to exert antibacterial effects, while DMOG is continuously released from the LDHs to promote long-term osteogenesis and angiogenesis. Additionally, the surface coating of DMOG-loaded LDHs not only mechanically reinforced the 3D printed PLLA scaffolds but also enhanced the scaffolds’ osteogenic activity due to the release of Mg2+ from the LDHs. It is also noteworthy that we found that eugenol, DMOG, and LDHs synergistically promote cell proliferation, angiogenesis, and osteogenic differentiation in vitro, as well as accelerate in vivo vascularized bone formation. This work proposes a method for manufacturing 3D printed scaffolds with multiple drug release capabilities, facilitating bone repair.The related results were published in ACS Nano under the title “Versatile 3D Printing Scaffold with Spatiotemporal Release of Multiple Drugs for Bone Regeneration” (ACS Nano 2025, 19, 14, 13637–13653, IF=15.8).
Original link: https://doi.org/10.1021/acsnano.4c13265
Image Dispatch
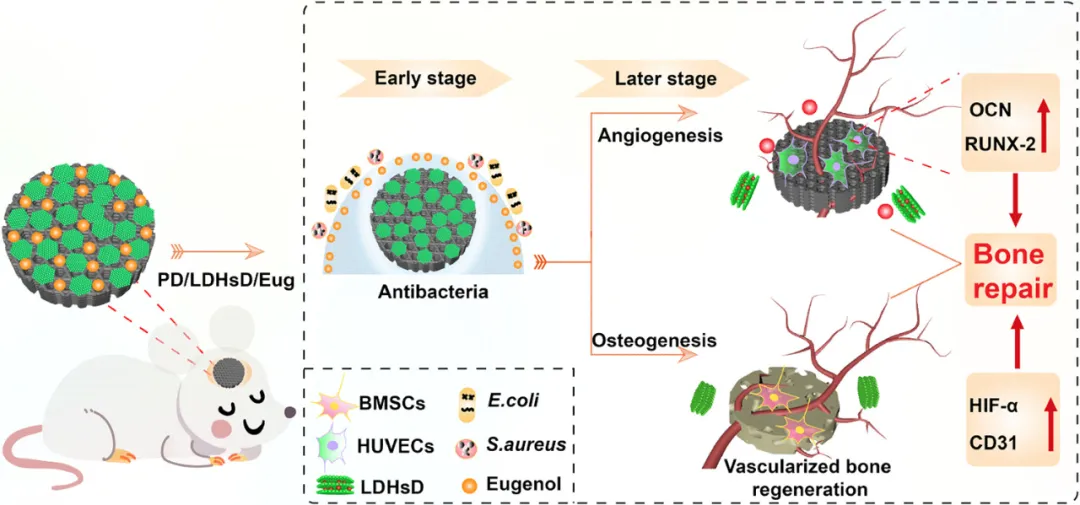
Figure 1. Schematic diagram of the spatiotemporal controlled release of DMOG and eugenol in the construction of multifunctional 3D printed PLLA composite scaffolds, exerting antibacterial effects in the early stages of in vivo cranial repair and promoting osteogenesis and angiogenesis in the later stages.
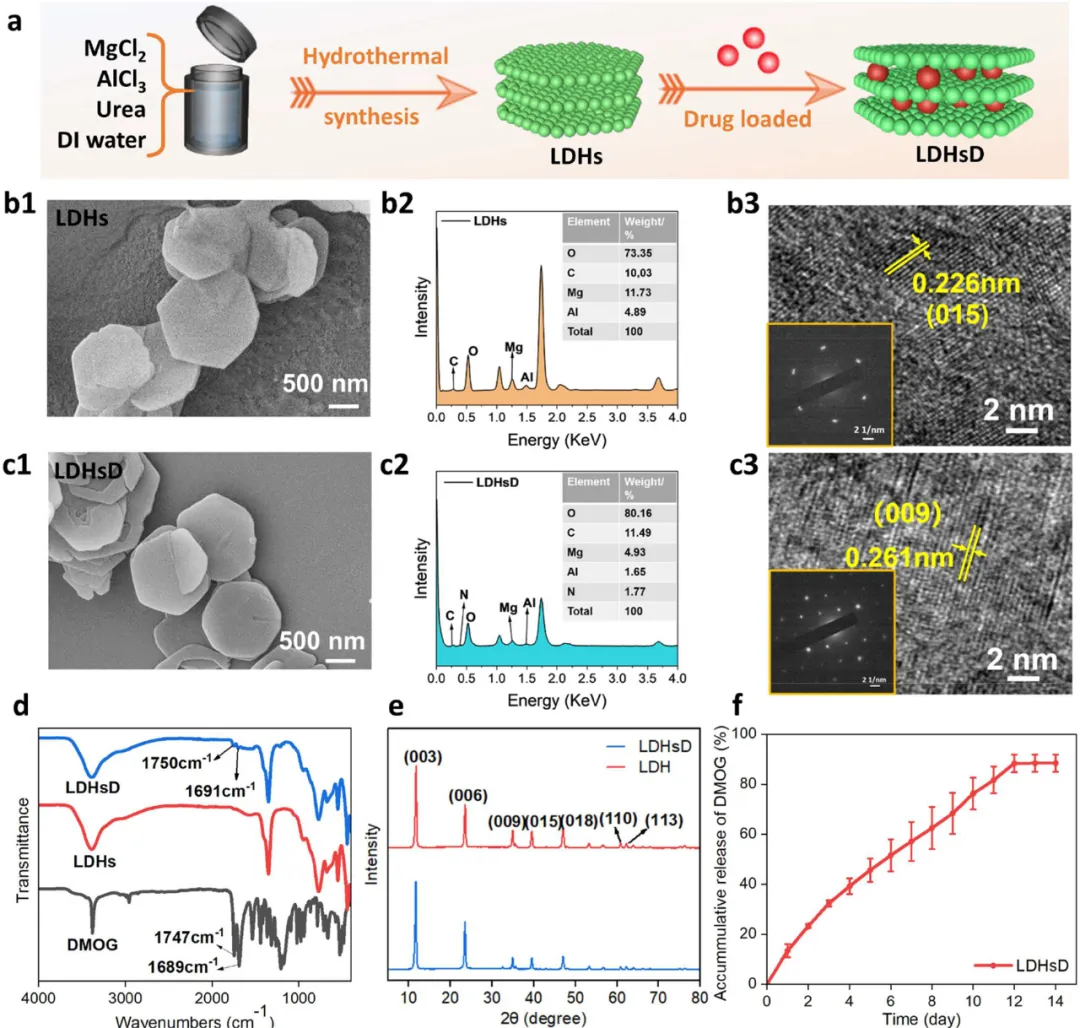
Figure 2. Regulation of the physicochemical properties of LDHs and LDHsD. (a) Schematic diagram of the preparation of LDHsD. (b1, c1) SEM images of LDHs and LDHsD, respectively. (b2, c2) EDS elemental analysis of LDHs and LDHsD. (b3, c3) HRTEM images and electron diffraction images of LDHs and LDHsD. (d) FT-IR spectra of LDHs, DMOG, and LDHsD. (e) XRD patterns of LDHs and LDHsD. (f) Drug release curve of DMOG from LDHsD.
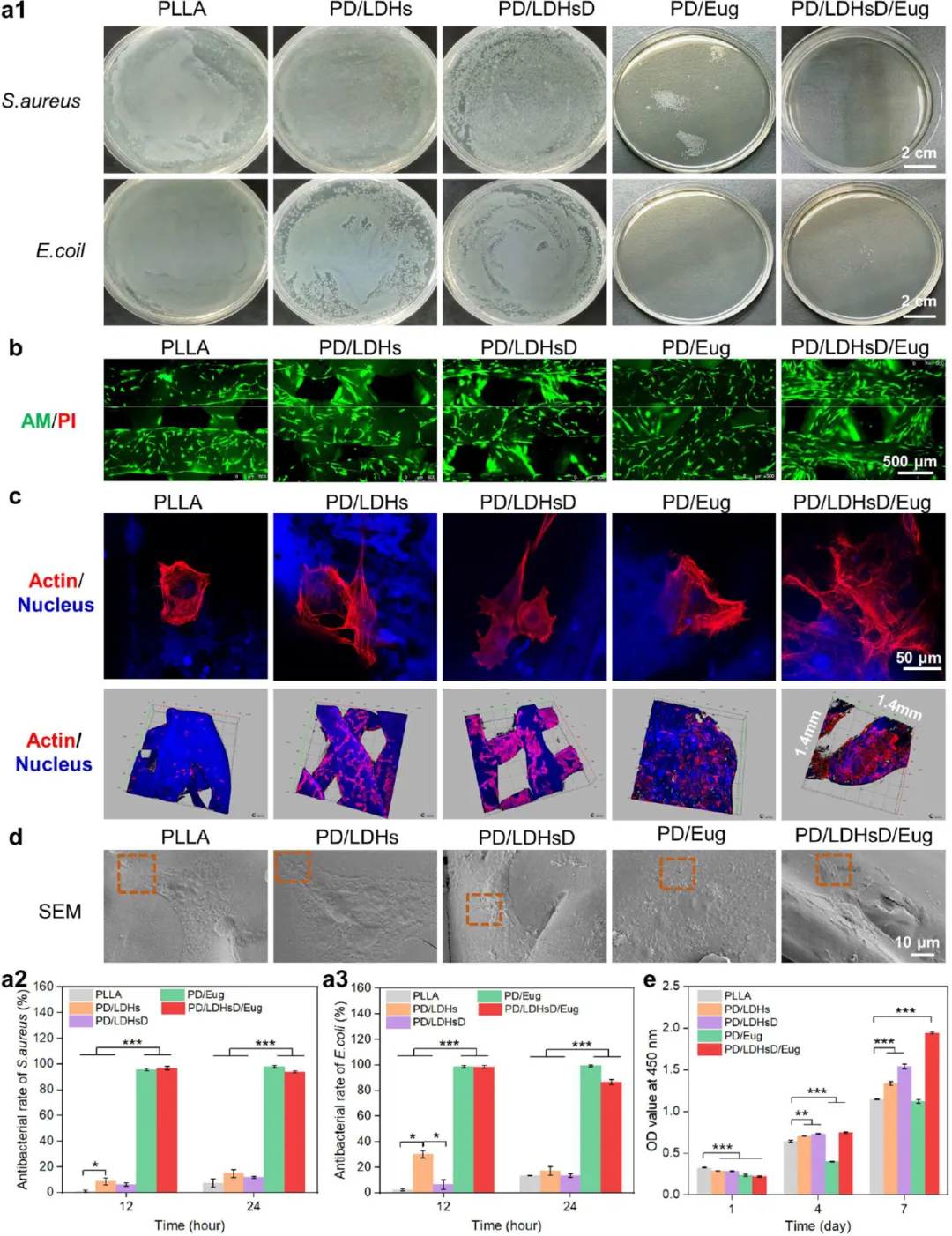
Figure 3. Characterization of raw PLLA and composite scaffolds. (a) Schematic diagram of the manufacturing of dual-loaded drug scaffolds through 3D printing. (b) Physical photos of six groups of scaffolds and pore structure images of PD/LDHsD/Eug composite scaffolds taken using a stereomicroscope. (c) Surface SEM images of six groups of scaffolds. (d1) Surface hydrophilicity of the scaffolds. (d2, d3) Compressive performance of the scaffolds under wet conditions. (e1−e3) XPS wide scan and fine spectra of PD/LDHs and PD/LDHsD/Eug, respectively. (f1, f2) Cumulative release curves of eugenol and metal ions from PD/LDHsD/Eug scaffolds (n =5, *p < 0.05, **p <0.01, ***p <0.001 by Student’s t-test).
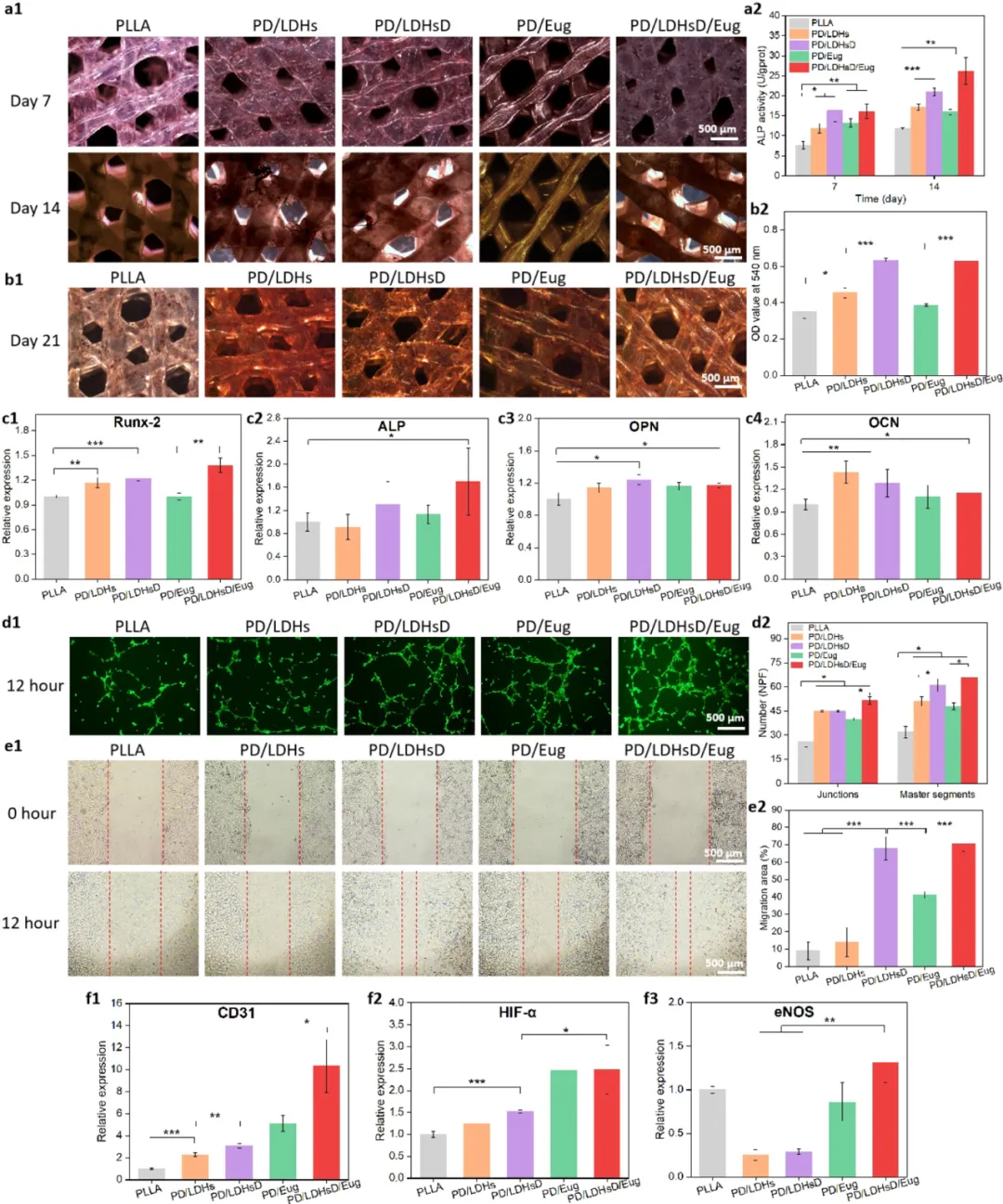
Figure 4. Evaluation of the antibacterial activity and cell compatibility of the composite scaffolds in vitro. (a1) Colony photos of E. coli and S. aureus cultured with the scaffolds for 24 hours. (a2, a3) Subsequently, the scaffolds were cultured with E. coli and S. aureus for 12 and 24 hours, respectively, with statistical graphs of the antibacterial rates for each group of scaffolds. (b) Fluorescence images of BMSCs stained with AM/PI on the scaffolds after 7 days. (c) Confocal images of BMSCs cultured on the scaffolds for 72 hours, with the cell cytoskeleton treated with rhodamine-phalloidin (red) and the cell nucleus stained with DAPI (blue) (topology is the two-dimensional topological image of the cells, and the bottom is the distribution image of the cells growing on the scaffolds). (d) SEM images of BMSCs cultured on the scaffolds for 24 hours. (e) Measurement of BMSCs proliferation ability through CCK-8 after 1, 4, and 7 days of incubation on the scaffolds.

Figure 5. Evaluation of the osteogenic and angiogenic activities of the scaffolds. (a1, a2) ALP staining images and quantitative results of BMSCs cultured on five groups of scaffolds for 7 and 14 days, respectively. (b1, b2) Qualitative images of Alizarin red staining and quantitative results of BMSCs incubated on the scaffolds for 21 days. (c1−c4) Expression analysis of relevant osteogenic genes (Runx-2, ALP, OPN, and OCN) after BMSCs were cultured on the scaffolds for 14 days. (d1) Images obtained from tube formation of HUVECs on scaffolds stained with AM. (d2) ImageJ was used to calculate the number of nodes and main segments in the tube formation experiment. (e1) Images of HUVECs at 0 and 12 hours in the scratch healing assay. (e2) Calculation of the cell migration rate of the scaffolds based on the ratio of the area migrated by cells to the scratch. (f1−f3) Expression of relevant angiogenic genes (CD31, HIF-α, and eNOS) detected by RT-PCR after HUVECs were cultured on the scaffolds for 5 days.
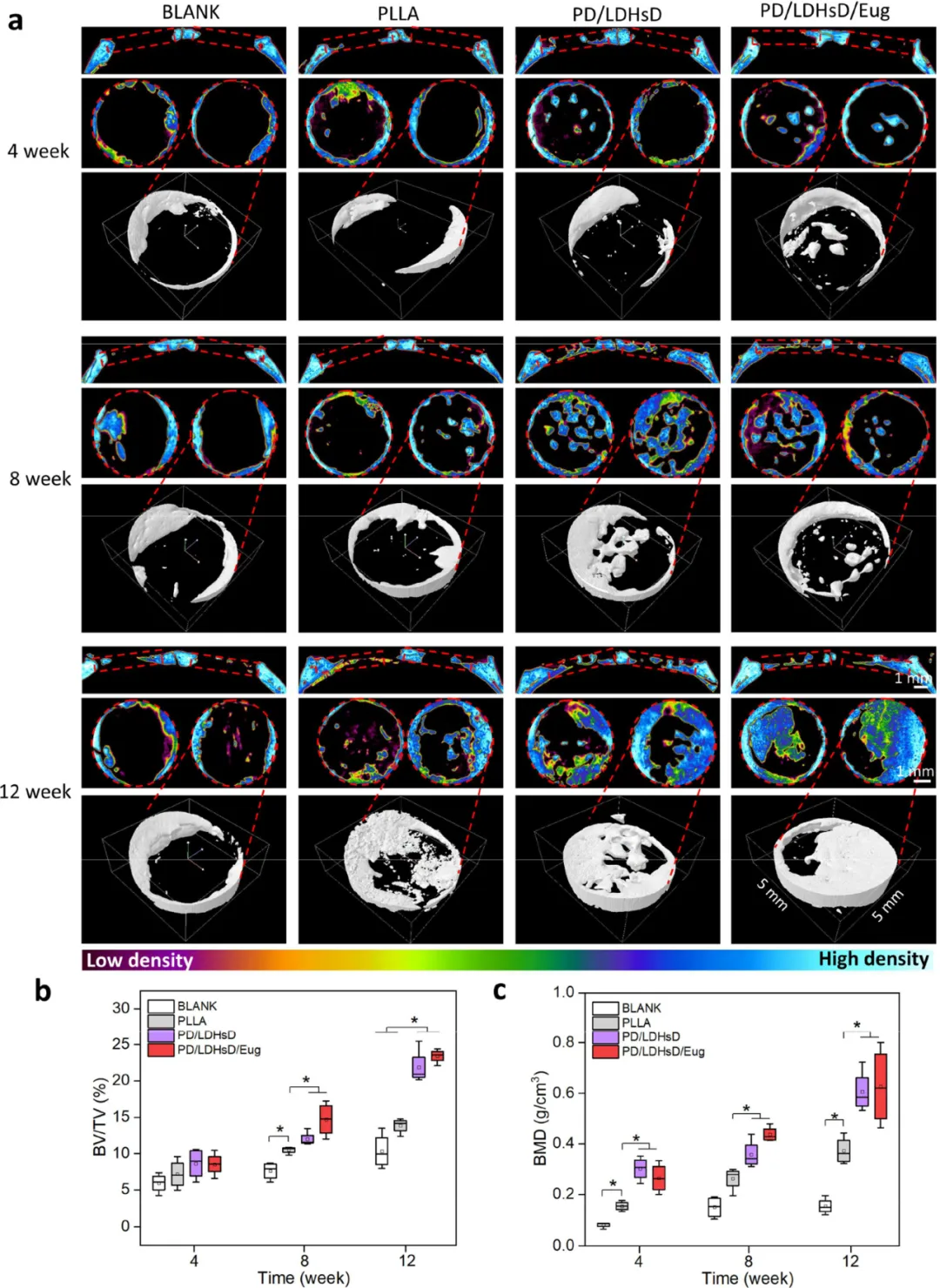
Figure 6. Micro-CT evaluation of cranial regeneration after scaffold implantation at 4, 8, and 12 weeks post-surgery. (a) Micro-CT reconstruction images of the region of interest in the defect area. (b, c) Quantitative analysis of BV/TV and BMD.
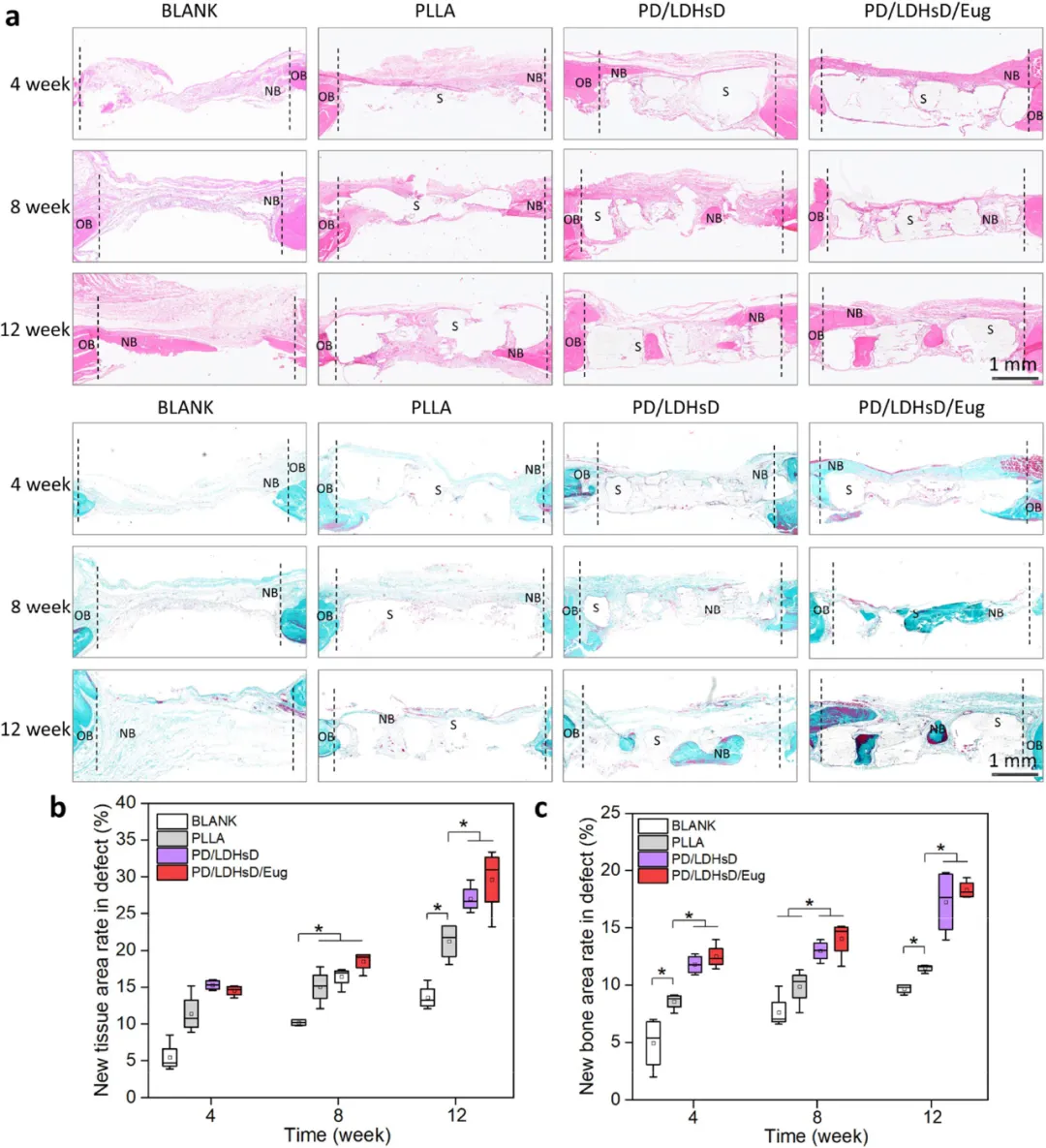 Figure 7. Histological staining evaluation of bone defect areas after scaffold implantation at different time points. (a) H&E staining and Goldner’s trichrome staining analysis of different groups. (b,c) Quantitative analysis of H&E and Goldner’s trichrome staining.
Figure 7. Histological staining evaluation of bone defect areas after scaffold implantation at different time points. (a) H&E staining and Goldner’s trichrome staining analysis of different groups. (b,c) Quantitative analysis of H&E and Goldner’s trichrome staining.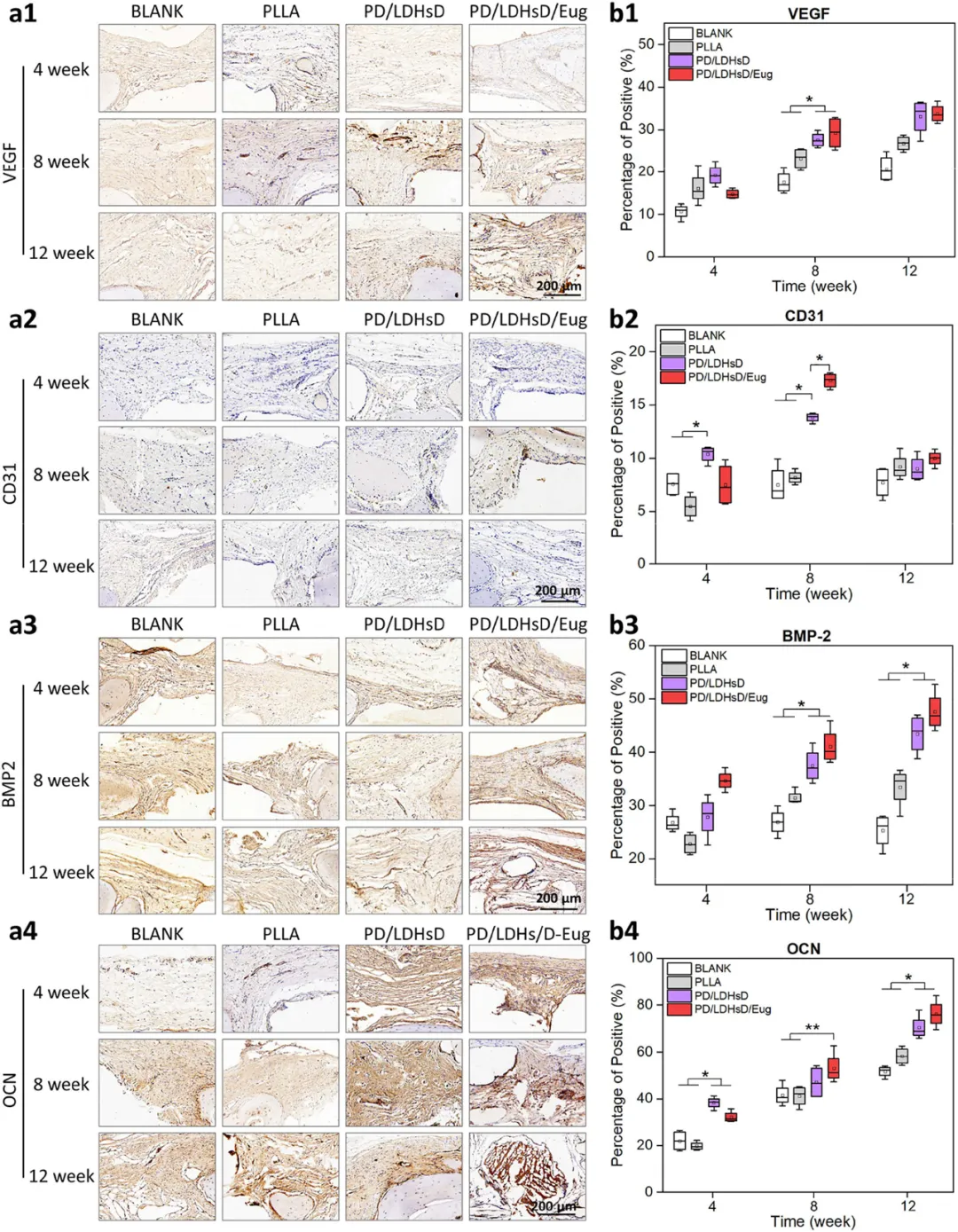 Figure 8. Histological evaluation of bone defect repair in a critical-sized cranial defect rat model after scaffold implantation at different time points. (a1−a4) Representative IHC staining and (b1−b4) statistical analysis of the percentage of positive areas (brown areas) for angiogenic markers (VEGF and CD31) and osteogenic markers (BMP-2 and OCN).
Figure 8. Histological evaluation of bone defect repair in a critical-sized cranial defect rat model after scaffold implantation at different time points. (a1−a4) Representative IHC staining and (b1−b4) statistical analysis of the percentage of positive areas (brown areas) for angiogenic markers (VEGF and CD31) and osteogenic markers (BMP-2 and OCN).
Conclusion
In summary, we successfully loaded eugenol and LDHsD onto the surface of 3D printed PLLA scaffolds through PDA layers. Due to the early release of eugenol, the scaffolds initially exhibited strong antibacterial effects, while the continuous release of DMOG from LDHs provided long-term support for osteogenesis and angiogenesis in the later stages. Furthermore, surface modification using DMOG-loaded LDHs effectively enhanced the mechanical properties of the 3D printed PLLA scaffolds. Importantly, the presence of LDHs also promoted the osteogenic activity of the scaffolds by releasing Mg2+, which can synergistically stimulate angiogenesis and osteogenic differentiation with DMOG and eugenol. The prepared PD/LDHsD/Eug scaffold demonstrated good bone regeneration in a rat cranial defect model due to the synergistic effects of LDHs, DMOG, and eugenol. This study developed a strategy for simultaneously modifying antibacterial agents and osteogenic and angiogenic drugs on scaffolds, and more importantly, the spatiotemporal controlled release of drugs in response to the bone tissue repair process is expected to become a very effective construction method for bone tissue engineering scaffolds.
Disclaimer: For academic exchange only, this platform does not advocate the copyright of the original text. If there is any infringement, please contact us for deletion. The editor’s level is limited, and if there are omissions in the literature interpretation or author introduction, we sincerely apologize. Please contact the author team in a timely manner, and we will make corrections as soon as possible. Thank you for your understanding!
Experts and scholars are welcome to promote their research achievements at Nanjing Jena Science. Submission email: [email protected]. Please include detailed contact information, including school or company name, name, and email address when submitting. We will publish and promote promptly.