The cancer-associated fibroblast (CAF) barrier in pancreatic ductal adenocarcinoma (PDAC) significantly restricts clinical prognosis. The main obstacles in PDAC treatment include limited immune cell and drug penetration, as well as an immunosuppressive microenvironment. Based on this, Yongjun Liu and Na Zhang from Shandong University reported a “shooting fish in a barrel” strategy by preparing a lipid-polymer hybrid drug delivery system (PI/JGC/L-A), transforming it into a “barrel” with antitumor drug depot characteristics, thereby overcoming the CAF barrier, alleviating the immunosuppressive microenvironment, and increasing immune cell infiltration (Figure 1). The strategy of converting the CAF barrier into an antitumor drug depot represents a promising approach for PDAC and may benefit any tumor facing drug delivery obstacles. The relevant research findings were published on June 16, 2023, in ACS NANO under the title “Transforming Cancer-Associated Fibroblast Barrier into Drug Depots to Boost Chemo-Immunotherapy in ‘Shooting Fish in a Barrel’ Pattern”.
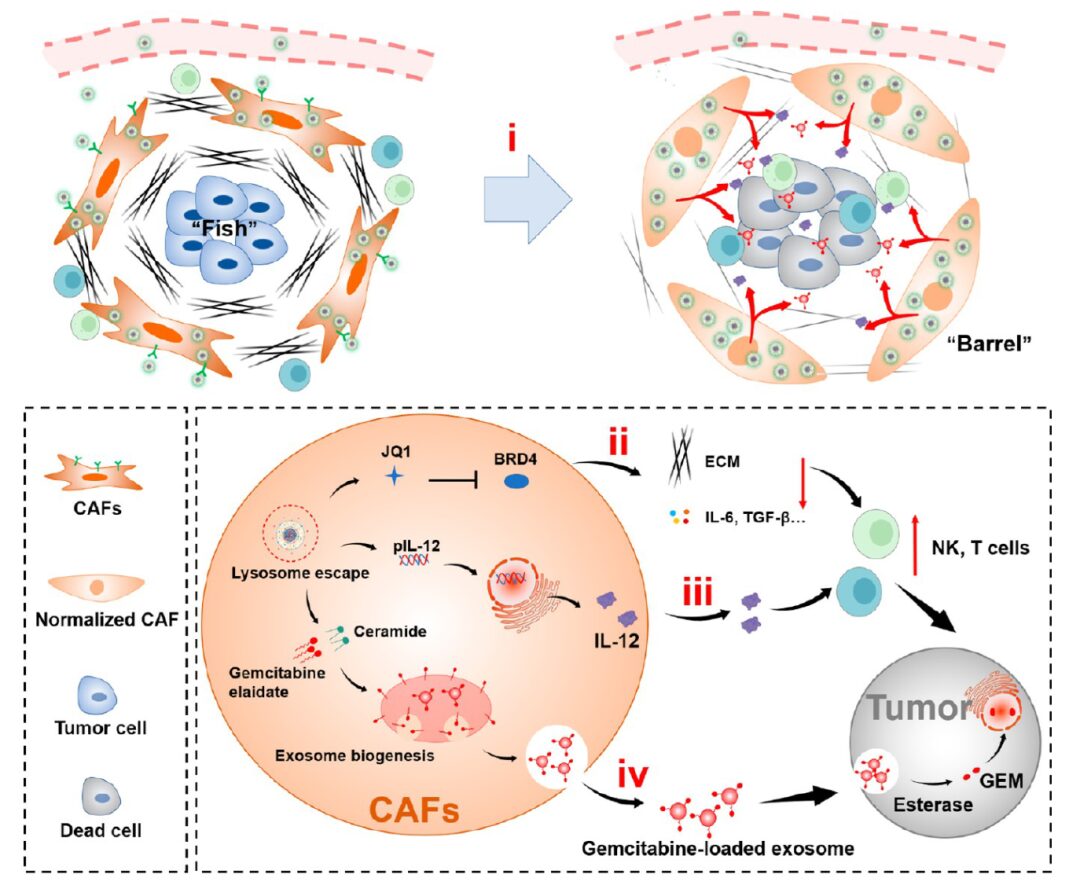
Figure 1 Schematic of PI/JGC/L-A: enabling drug penetration through the CAF barrier, increasing immune cell infiltration at the tumor site, and promoting chemotherapeutic immunotherapy in a “shooting fish in a barrel” pattern.
1. Preparation and Characterization of PI/JGC/L-A
The preparation process for PI/JGC/L-A nanoparticles for CAF solidification is shown in Figure 2a. The nanoparticles consist of a PI core and a JGC/L-A liposome shell. The increase in particle size and decrease in negative potential of PI/JGC/L-A nanoparticles observed by differential scanning calorimetry and transmission electron microscopy indicate successful preparation (Figure 2b).
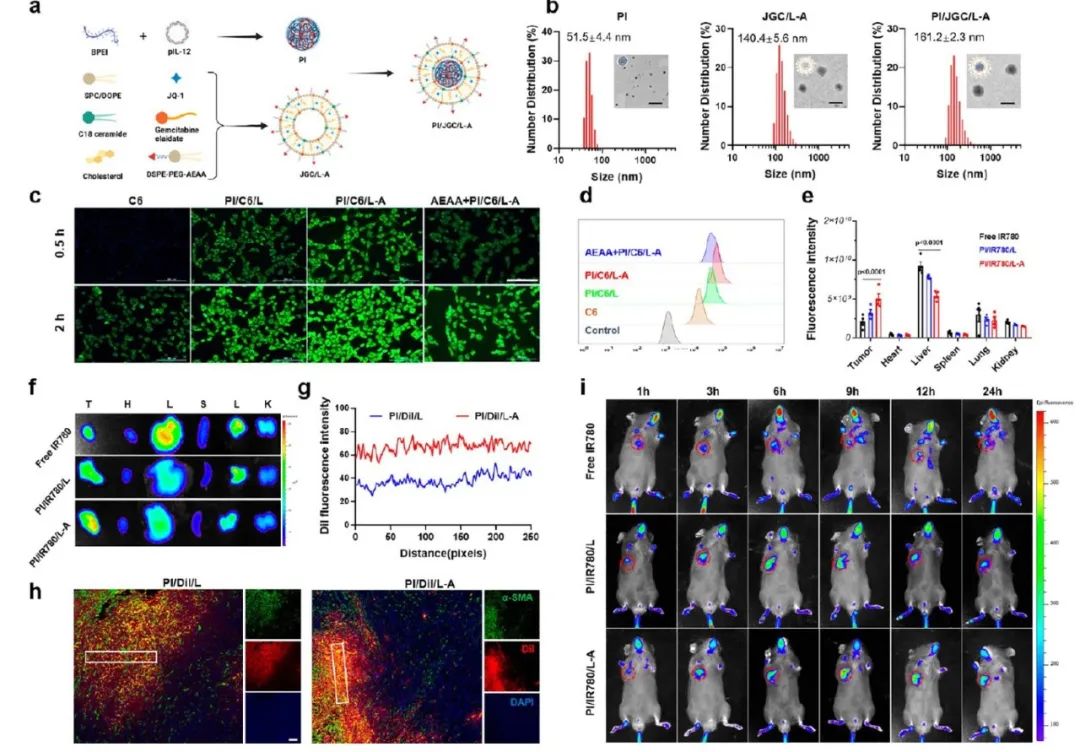
Figure 2 Preparation process, characterization, enhanced CAF targeting, and CAF accumulation ability of PI/JGC/L-A.
2. Targeting and Accumulation of PI/JGC/L-A in CAF
The CAF barrier acts as a drug depot, and the amount of drug accumulated in CAF is closely related to its antitumor effects. Increasing drug accumulation levels in CAF can provide a richer source for antitumor drugs. The C6 fluorescence intensity in the PI/C6/L-A group was higher than that in the PI/C6/L group (Figure 2c, d), indicating greater uptake by CAF. Further evaluation of the CAF targeting and in vivo accumulation of PI/JGC/L-A was conducted. IR780 formulation was intravenously injected into subcutaneous PDAC tumor model animals. As shown in Figures 2e, f, and i, the tumors in the PI/IR780/L-A group exhibited stronger fluorescence intensity, approximately 1.67 times that of the unmodified AEAA nanoparticles, indicating that AEAA facilitated tumor accumulation of PI/IR780/L-A. PI/DIL/L-A enhanced DIL signal in tumor tissue slices, indicating CAF barrier accumulation (Figure 2g, h). CAFs captured a large number of nanoparticles, with few reaching the tumor site, which largely limited the therapeutic effect. However, in the current design, we utilized the BSB effect to actively target and increase nanoparticle accumulation in CAF, aiding in the sustained release of antitumor drugs at the tumor site.
3. Normalization of PI/JGC/L-A CAF while Maintaining Viability to Create a “Barrel”
When the CAF barrier is used as a drug depot, its viability must be ensured. The authors first determined the esterase activity of CAF and Panc02 cells using the fluorescein diacetate (FDA) method. Higher fluorescence intensity was observed in tumor cells, indicating that the esterase activity of Panc02 cells was higher than that of CAF (Figure 3a, b). The optimal dose of JQ1 for CAF was then preliminarily studied. CAF was cultured with different JQ1-containing formulations, followed by confocal microscopy and Western blot analysis to determine its normalization degree. The results indicated that only JQ1, PI/JGC/L, and PI/JGC/L-A could inhibit the expression of α-SMA and type I collagen, inducing significant lipid droplet formation, indicating CAF normalization (Figure 3d, f). The Western blot results showed a similar pattern (Figure 3e). In summary, these results indicate that PI/JGC/L-A can normalize CAF without eliminating them, effectively reducing the risk of tumor metastasis and preventing stronger immunosuppression at the tumor site.
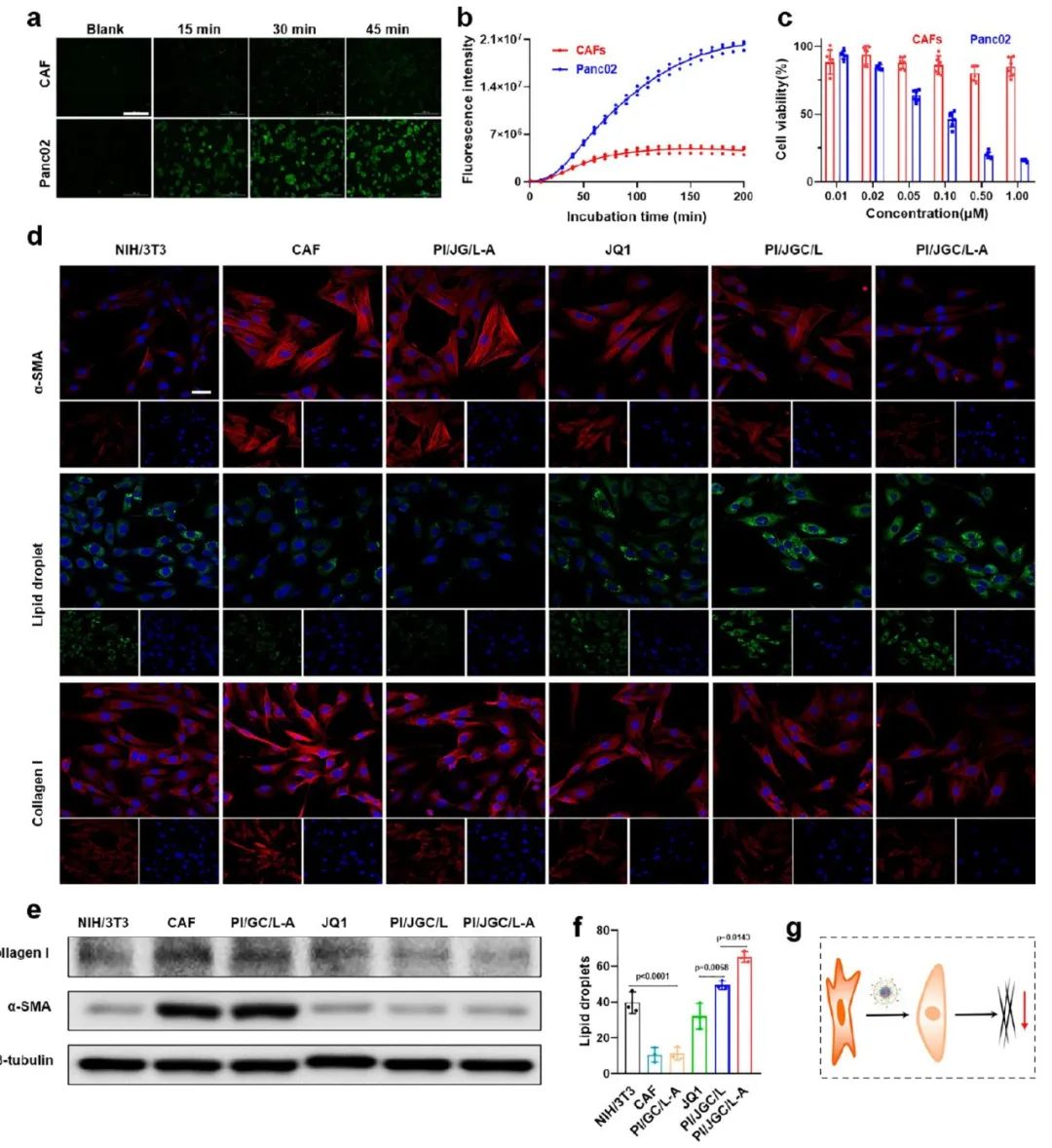
Figure 3 Normalization of PI/JGC/L-A in CAF to create a “barrel” while maintaining viability.
4. PI/JGC/L-A Modulates “Shooting Fish in a Barrel”
To examine the effects of JQ1 and pIL-12 on the immunosuppressive microenvironment, the authors conducted in vitro immune cell experiments, as shown in Figure 4b. In the JQ1 pretreated CAF medium, the expression of immunosuppressive cytokines (such as TGF-β and IL-6) was significantly reduced (Figure 4c, d). The proportion of NK cells (CD3−NK1.1+) was restored, while the proportion of exhausted T cells (CD3+CD8+PD-1+) decreased. When pIL-12 was included, the number of T cells and NK cells significantly increased. When JQ1 and pIL-12 acted simultaneously, T cells and NK cells showed the greatest proliferation, with the CD8+PD-1+ T cell phenotype reduced to the lowest level (Figure 4e-j). After co-culturing pretreated immune cells with Panc02 cells, the Panc02 cell survival rate in the PI/J/LA group was the lowest, indicating the strongest cytotoxicity of these immune cells (Figure 4k). All these results indicate thatJQ1 alleviated the immunosuppressive microenvironment, while IL-12 facilitated immune cell proliferation, and their combination effectively activated antitumor immunity at the tumor site.
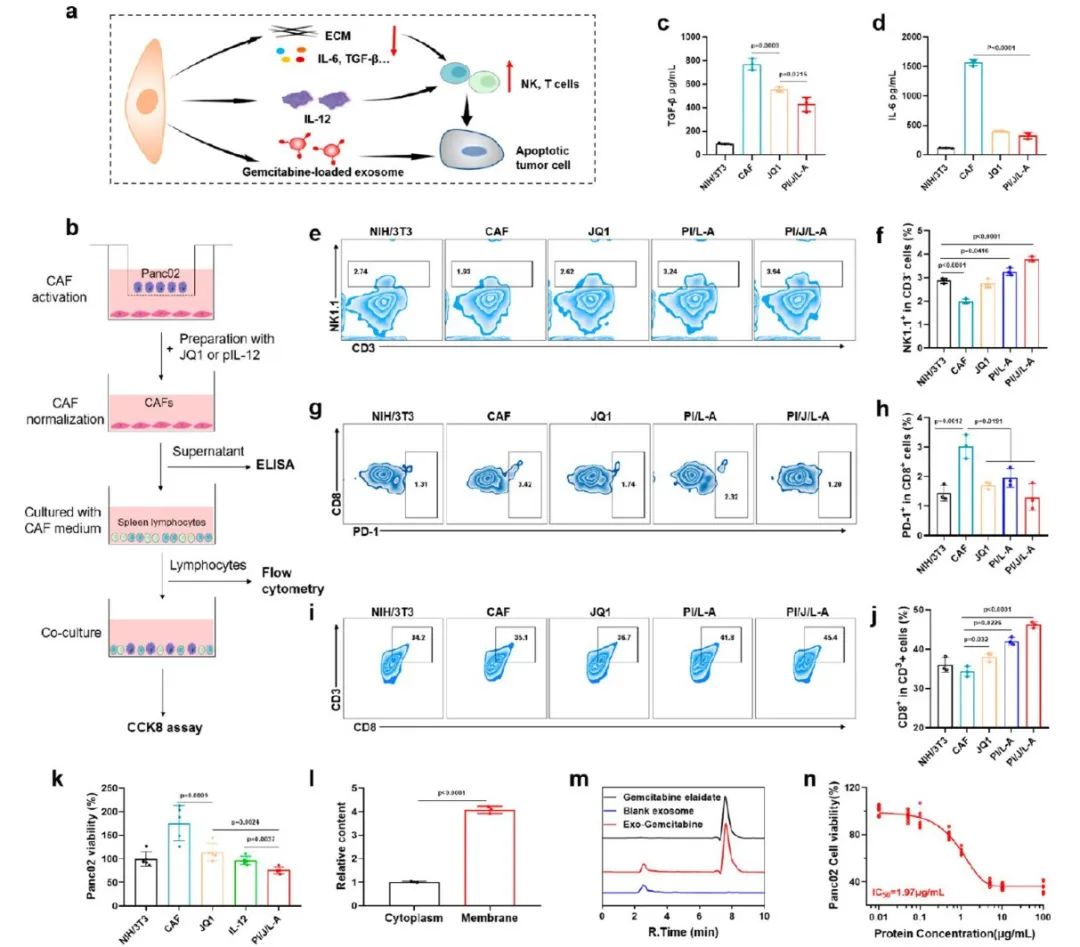
Figure 4 PI/JGC/L-A reduces cytokine-induced immunosuppression by normalizing CAF, activates immune cells by secreting IL-12, and generates exosomes loaded with gemcitabine for targeted PDAC chemotherapeutic immunotherapy.
5. In Vivo Antitumor Efficacy
Through the establishment of subcutaneous models and in situ PDAC models, the antitumor effects of PI/JGC/L-A were studied (Figures 5 and 6). The results indicated that PI/JGC/L-A could completely inhibit tumor growth, possibly due to CAF normalization promoting immune cell infiltration and converting CAF into a drug depot to enhance chemotherapy and immunotherapy.
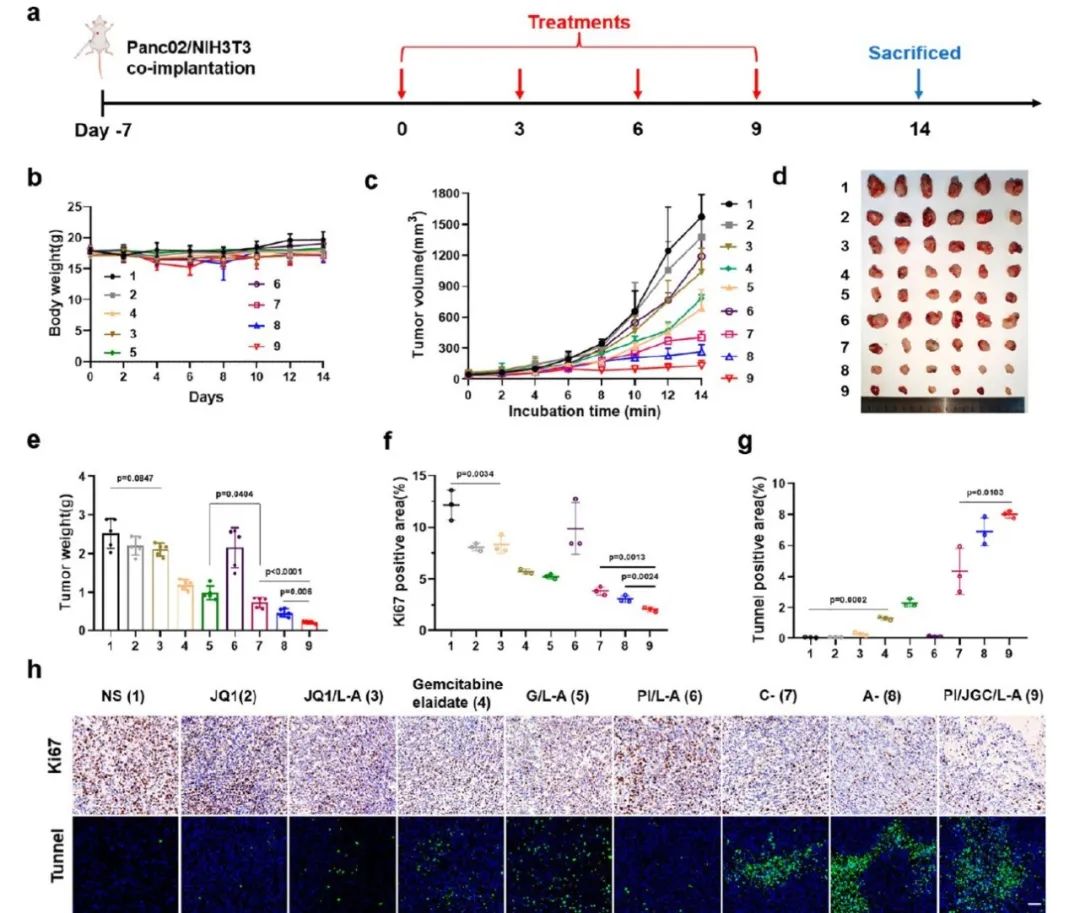
Figure 5 PI/JGC/L-A significantly improves antitumor effects in subcutaneous PDAC tumor models.
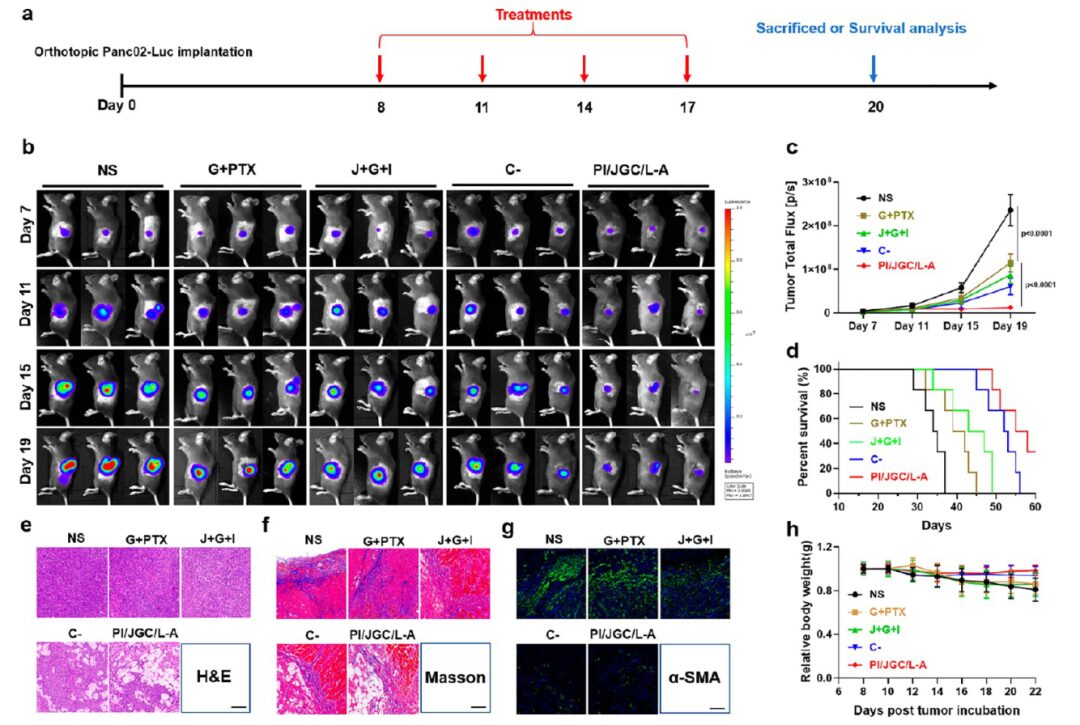
Figure 6 PI/JGC/L-A inhibits tumor growth, prolongs survival, and reshapes the TME in in situ PDAC tumor models.
In summary, in this study, the constructed nanoparticle drug PI/JGC/L-A normalized CAF to a “barrel”, modulated the “barrel” to secrete gemcitabine-loaded exosomes and IL-12 deep into the tumor, ultimately achieving chemotherapeutic immunotherapy in the “shooting fish in a barrel” strategy. This study did not eliminate therapeutic obstacles but rather acted upon them. This strategy is an effective method to overcome drug delivery obstacles and may be applicable to any tumor model facing such obstacles, including but not limited to PDAC.
Source:
https://pubs.acs.org/doi/10.1021/acsnano.3c02272
Join Our Group for Discussion
Regarding the research direction of regenerative medicine, the EFL public account has established an “Academic Exchange Group“, scan the QR code below to add the editor’s WeChat for group discussion~