Sonodynamic therapy (SDT) is a non-invasive treatment method based on reactive oxygen species (ROS) that interacts with ultrasound (US) activated sonosensitizers and surrounding oxygen and water molecules to produce highly cytotoxic ROS and induce apoptosis. Due to its ability to penetrate deep tissues while causing minimal damage to normal cells, it has significant potential in tumor treatment. Although SDT has developed rapidly and achieved great success in recent years, the complex tumor microenvironment (tumor microenvironment, TME) and excessive reductive substances within the TME, such as glutathione (glutathione, GSH), may protect cancer cells from high oxidative stress, thereby inhibiting its sensitization effect, which further weakens the therapeutic efficacy of SDT. Therefore, developing novel sonosensitizers that can simultaneously regulate the TME and amplify tumor oxidative stress may be a more effective approach to improve SDT-mediated cancer therapy.
 Based on this, Professor Cheng Liang from the Institute of Functional Nano and Soft Materials, Suzhou University prepared a novel oxygen-deficient molybdenum oxide (MoOX) nanoscale material as a new nanosensitizer and TME stimulator for ultrasound (US) enhanced tumor metalloimmunotherapy (Schematic Diagram 1).After polyethylene glycol modification, MoOX-PEG exhibited efficient reactive oxygen species (ROS) generation and glutathione (GSH) consumption capacity. Under US irradiation, MoOX-PEG generated a large amount of ROS to induce cancer cell damage and strong immunogenic cell death (ICD), effectively inhibiting tumor growth. More importantly, MoOX-PEG itself further stimulated the maturation of dendritic cells (DC) and triggered the activation of the cGAS-STING pathway to enhance immune effects. Due to the strong ICD induced by SDT and the efficient DC maturation induced by MoOX-PEG, the combination therapy of MoOX triggered SDT and aCTLA-4 further amplified the antitumor treatment, inhibited cancer metastasis, and elicited a robust immune response, effectively defeating distant tumors. The relevant work was published in Angew under the title “Oxygen-deficient Molybdenum Oxide Nanosensitizers for Ultrasound-enhanced Cancer Metalloimmunotherapy.”
Based on this, Professor Cheng Liang from the Institute of Functional Nano and Soft Materials, Suzhou University prepared a novel oxygen-deficient molybdenum oxide (MoOX) nanoscale material as a new nanosensitizer and TME stimulator for ultrasound (US) enhanced tumor metalloimmunotherapy (Schematic Diagram 1).After polyethylene glycol modification, MoOX-PEG exhibited efficient reactive oxygen species (ROS) generation and glutathione (GSH) consumption capacity. Under US irradiation, MoOX-PEG generated a large amount of ROS to induce cancer cell damage and strong immunogenic cell death (ICD), effectively inhibiting tumor growth. More importantly, MoOX-PEG itself further stimulated the maturation of dendritic cells (DC) and triggered the activation of the cGAS-STING pathway to enhance immune effects. Due to the strong ICD induced by SDT and the efficient DC maturation induced by MoOX-PEG, the combination therapy of MoOX triggered SDT and aCTLA-4 further amplified the antitumor treatment, inhibited cancer metastasis, and elicited a robust immune response, effectively defeating distant tumors. The relevant work was published in Angew under the title “Oxygen-deficient Molybdenum Oxide Nanosensitizers for Ultrasound-enhanced Cancer Metalloimmunotherapy.”
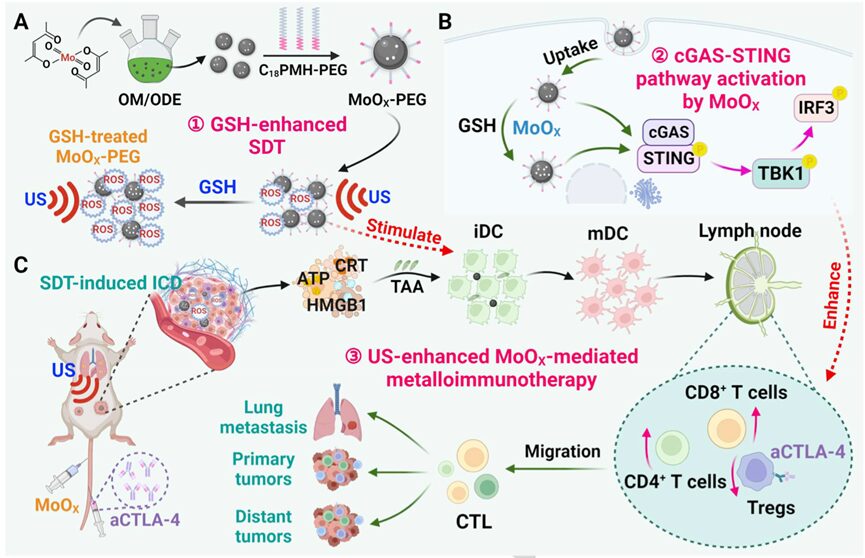 Schematic Diagram 1. Oxygen-deficient molybdenum nanosensitizers for ultrasound-enhanced cancer metalloimmunotherapy
The researchers prepared ultra-small molybdenum oxide nanoparticles (MoOX NPs) via a high-temperature decomposition method. They mixed oleylamine (OM) and 1-octadecene (ODE) solvent with molybdenum acetylacetonate, heated the mixture to 140℃ to remove any low-boiling-point solvents, and further heated to 320 ℃ for 30 minutes (Figure 1A). Transmission electron microscopy (TEM) images showed that the MoOX NPs had a uniform structure with an average size of (3.42±0.37)nm (Figure 1B-C). Based on high-resolution TEM, the lattice spacing was measured to be ~ 0.197 nm (Figure 1D). Additionally, the Mo and O elements in MoOX NPs were evenly distributed in the energy spectrum (EDS) images (Figure 1E&J). From Raman characterization (Figure 1F), the characteristic peak at ~ 969 cm−1 corresponds to the stretching vibration of terminal oxygen (Mo-O), while the peaks at ~ 835 and ~799 cm−1 correspond to the stretching mode of bidentate bridging oxygen (Mo2-O), and the peaks at ~711 cm−1 and ~479 cm−1 correspond to the stretching mode of tridentate oxygen (Mo3-O). The higher Raman peak wavenumbers may be attributed to the generation of oxygen vacancies in MoOXNPs.
Schematic Diagram 1. Oxygen-deficient molybdenum nanosensitizers for ultrasound-enhanced cancer metalloimmunotherapy
The researchers prepared ultra-small molybdenum oxide nanoparticles (MoOX NPs) via a high-temperature decomposition method. They mixed oleylamine (OM) and 1-octadecene (ODE) solvent with molybdenum acetylacetonate, heated the mixture to 140℃ to remove any low-boiling-point solvents, and further heated to 320 ℃ for 30 minutes (Figure 1A). Transmission electron microscopy (TEM) images showed that the MoOX NPs had a uniform structure with an average size of (3.42±0.37)nm (Figure 1B-C). Based on high-resolution TEM, the lattice spacing was measured to be ~ 0.197 nm (Figure 1D). Additionally, the Mo and O elements in MoOX NPs were evenly distributed in the energy spectrum (EDS) images (Figure 1E&J). From Raman characterization (Figure 1F), the characteristic peak at ~ 969 cm−1 corresponds to the stretching vibration of terminal oxygen (Mo-O), while the peaks at ~ 835 and ~799 cm−1 correspond to the stretching mode of bidentate bridging oxygen (Mo2-O), and the peaks at ~711 cm−1 and ~479 cm−1 correspond to the stretching mode of tridentate oxygen (Mo3-O). The higher Raman peak wavenumbers may be attributed to the generation of oxygen vacancies in MoOXNPs.
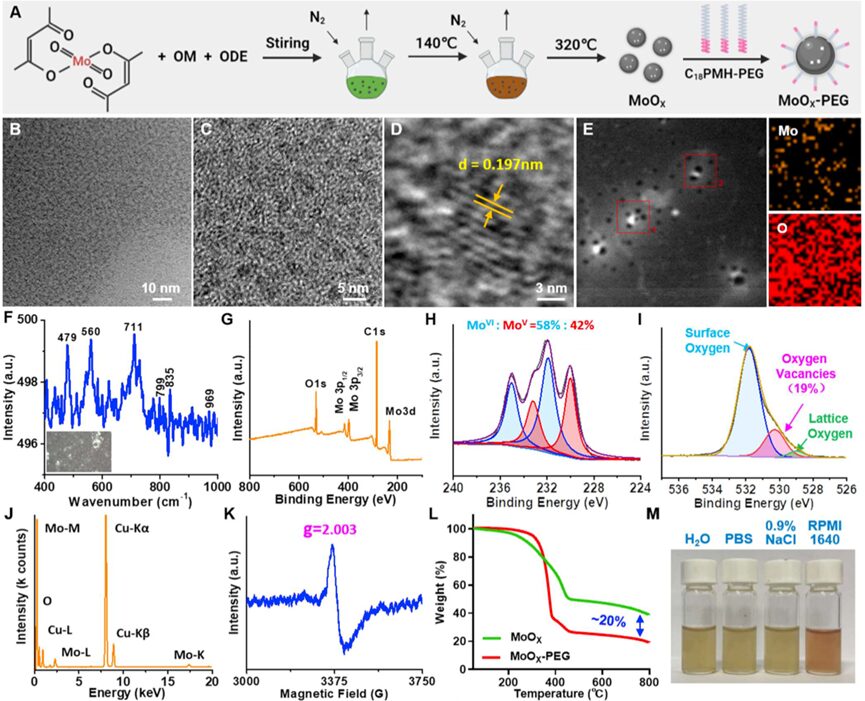 Figure 1. Preparation and characterization of MoOX NPs
MoO3 has been widely studied as an excellent photocatalyst. The synthesized MoOX nanoparticles possess abundant oxygen vacancies and suitable band gaps, making them good ultrasound-triggered SDT catalysts (Figure 2A). To investigate whether MoOX-PEG NPs can enhance ultrasound catalysis, the researchers used 1,3-diphenylisobenzofuran (DPBF) probe to detect the generation of singlet oxygen (1O2) under ultrasound irradiation (Figure 2B). Interestingly, as the US irradiation time increased, the UV-vis absorption of DPBF at ~ 420 nm significantly decreased, indicating effective1O generation (Figure 2B). The oxidation rate of DPBF under hypoxic MoOX + US irradiation showed concentration and power density dependence (Figure 2C and Figure S2), and was much better than traditional sonosensitizers like titanium dioxide (Figure S3). Similar to the DPBF probe, the 9,10-diphenylanthracene (DPA) probe was also used to evaluate the sonodynamic performance of MoOX nanoparticles. The researchers found that the DPA probe also showed significant decrease under US irradiation (Figure 2D), indicating effective ROS generation. Additionally, ESR detection further confirmed that MoOX + US treatment exhibited the highest levels of1O2 (Figure 2E), indicating that MoOX can serve as an excellent nanosensitizer for cancer treatment.
Figure 1. Preparation and characterization of MoOX NPs
MoO3 has been widely studied as an excellent photocatalyst. The synthesized MoOX nanoparticles possess abundant oxygen vacancies and suitable band gaps, making them good ultrasound-triggered SDT catalysts (Figure 2A). To investigate whether MoOX-PEG NPs can enhance ultrasound catalysis, the researchers used 1,3-diphenylisobenzofuran (DPBF) probe to detect the generation of singlet oxygen (1O2) under ultrasound irradiation (Figure 2B). Interestingly, as the US irradiation time increased, the UV-vis absorption of DPBF at ~ 420 nm significantly decreased, indicating effective1O generation (Figure 2B). The oxidation rate of DPBF under hypoxic MoOX + US irradiation showed concentration and power density dependence (Figure 2C and Figure S2), and was much better than traditional sonosensitizers like titanium dioxide (Figure S3). Similar to the DPBF probe, the 9,10-diphenylanthracene (DPA) probe was also used to evaluate the sonodynamic performance of MoOX nanoparticles. The researchers found that the DPA probe also showed significant decrease under US irradiation (Figure 2D), indicating effective ROS generation. Additionally, ESR detection further confirmed that MoOX + US treatment exhibited the highest levels of1O2 (Figure 2E), indicating that MoOX can serve as an excellent nanosensitizer for cancer treatment.
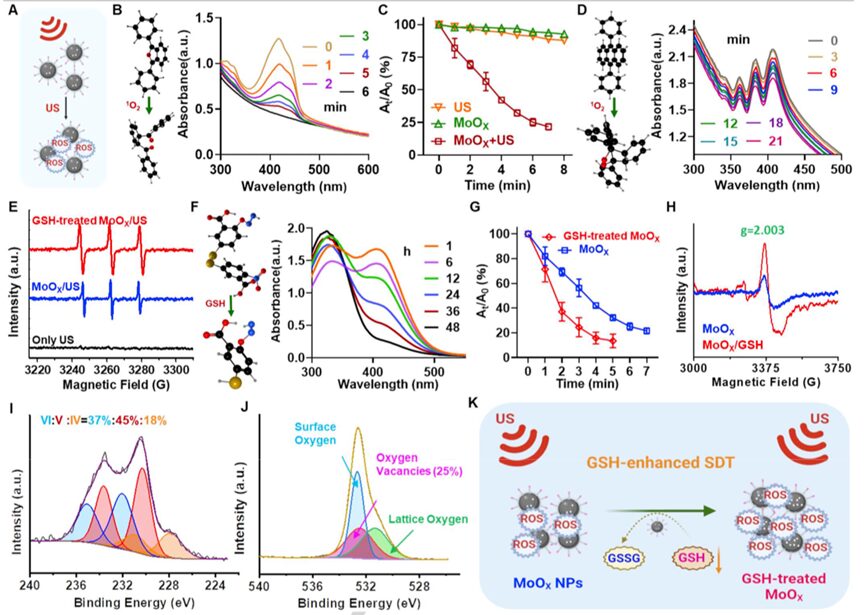 Figure 2. Sonodynamic kinetics and glutathione depletion evaluation of MoOX-PEG NPs
Encouraged by the significant sonodynamic properties of MoOX-PEG, the researchers evaluated the cytotoxic effects on cancer cells (Figure 3A). First, the researchers performed methyl thiazolyl tetrazolium (MTT) assays on mouse colon cancer CT26 cells, mouse breast cancer 4T1 cells, and human umbilical vein endothelial cells (HUVECs) after incubation with MoOX-PEG, finding that MoOX-PEG showed no significant toxicity to these cells (Figure 3B and Figure S9). Next, the researchers used 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine (DIR) dye-labeled MoOX PEG NPs to study the cellular uptake behavior. As the incubation time increased, the intracellular fluorescence signal gradually enhanced, reaching its strongest fluorescence level after 4 hours of incubation, indicating that MoOX PEG NPs exhibited efficient cellular endocytosis and nuclear escape (Figure 3E-F). The MTT method was used to detect the sonodynamic efficiency of MoOX-PEG; under US irradiation (40 kHz, 3 W/cm2), as the concentration of MoOX-PEG increased, the viability of 4T1 cells significantly decreased (Figure 3C), and under different US irradiation times, the cancer cells incubated with MoOX-PEG also showed significant cell death (Figure 3D). Using calcein AM (green represents live cells) and propidium iodide (PI, red represents dead cells) co-staining, the death ratio of 4T1 cells was evaluated after different treatments. The MoOX-PEG + US irradiation group showed significant red fluorescence, indicating good cytotoxic effects, while the MoOX-PEG incubation group or the simple US treatment group only showed a small amount of red fluorescence (Figure 3G). Similarly, flow cytometry results also demonstrated that the MoOX-PEG combined with US treatment exhibited the highest cytotoxic ability (Figure 3H). The above results indicate that the defect-type MoOX-PEG NPs exhibited significant sonocatalytic performance against cancer cells under ultrasound irradiation.
Figure 2. Sonodynamic kinetics and glutathione depletion evaluation of MoOX-PEG NPs
Encouraged by the significant sonodynamic properties of MoOX-PEG, the researchers evaluated the cytotoxic effects on cancer cells (Figure 3A). First, the researchers performed methyl thiazolyl tetrazolium (MTT) assays on mouse colon cancer CT26 cells, mouse breast cancer 4T1 cells, and human umbilical vein endothelial cells (HUVECs) after incubation with MoOX-PEG, finding that MoOX-PEG showed no significant toxicity to these cells (Figure 3B and Figure S9). Next, the researchers used 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine (DIR) dye-labeled MoOX PEG NPs to study the cellular uptake behavior. As the incubation time increased, the intracellular fluorescence signal gradually enhanced, reaching its strongest fluorescence level after 4 hours of incubation, indicating that MoOX PEG NPs exhibited efficient cellular endocytosis and nuclear escape (Figure 3E-F). The MTT method was used to detect the sonodynamic efficiency of MoOX-PEG; under US irradiation (40 kHz, 3 W/cm2), as the concentration of MoOX-PEG increased, the viability of 4T1 cells significantly decreased (Figure 3C), and under different US irradiation times, the cancer cells incubated with MoOX-PEG also showed significant cell death (Figure 3D). Using calcein AM (green represents live cells) and propidium iodide (PI, red represents dead cells) co-staining, the death ratio of 4T1 cells was evaluated after different treatments. The MoOX-PEG + US irradiation group showed significant red fluorescence, indicating good cytotoxic effects, while the MoOX-PEG incubation group or the simple US treatment group only showed a small amount of red fluorescence (Figure 3G). Similarly, flow cytometry results also demonstrated that the MoOX-PEG combined with US treatment exhibited the highest cytotoxic ability (Figure 3H). The above results indicate that the defect-type MoOX-PEG NPs exhibited significant sonocatalytic performance against cancer cells under ultrasound irradiation.
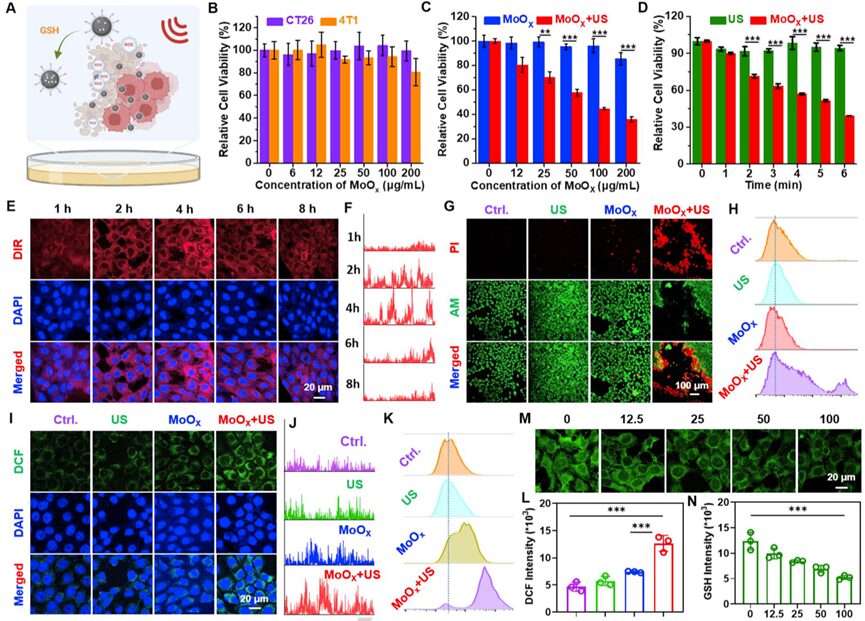 Figure 3. In vitro SDT of MoOX-PEG NPs
Previous studies have shown that ROS-mediated SDT can induce immunogenic cell death (immunogenic cell death, ICD), characterized by the upregulation of calreticulin (calreticulin, CRT) expression on the membrane of dying cancer cells, ATP (adenosine triphosphate) release, and activation of the immune system along with other endogenous adjuvants such as high mobility group box 1 (high mobility group box 1, HMGB1). Therefore, the researchers investigated whether MoOX-PEG NPs combined with US treatment could induce ICD based on the expression of CRT on the cell surface, the release of extracellular HMGB1, and the secretion of ATP. Immunofluorescence staining results showed (Figure 4B&C) that only the US or only the MoOX-PEG treatment slightly increased the expression of CRT and the release of HMGB1 in 4T1 cells. However, in the MoOX-PEG + US treatment group, both CRT expression and HMGB1 release were significantly upregulated. Furthermore, the ATP secretion of 4T1 cells was also significantly increased after MoOX-PEG combined with US treatment (Figure 4D). The above observations confirm that MoOX triggered SDT therapy can effectively induce ICD.
Figure 3. In vitro SDT of MoOX-PEG NPs
Previous studies have shown that ROS-mediated SDT can induce immunogenic cell death (immunogenic cell death, ICD), characterized by the upregulation of calreticulin (calreticulin, CRT) expression on the membrane of dying cancer cells, ATP (adenosine triphosphate) release, and activation of the immune system along with other endogenous adjuvants such as high mobility group box 1 (high mobility group box 1, HMGB1). Therefore, the researchers investigated whether MoOX-PEG NPs combined with US treatment could induce ICD based on the expression of CRT on the cell surface, the release of extracellular HMGB1, and the secretion of ATP. Immunofluorescence staining results showed (Figure 4B&C) that only the US or only the MoOX-PEG treatment slightly increased the expression of CRT and the release of HMGB1 in 4T1 cells. However, in the MoOX-PEG + US treatment group, both CRT expression and HMGB1 release were significantly upregulated. Furthermore, the ATP secretion of 4T1 cells was also significantly increased after MoOX-PEG combined with US treatment (Figure 4D). The above observations confirm that MoOX triggered SDT therapy can effectively induce ICD.
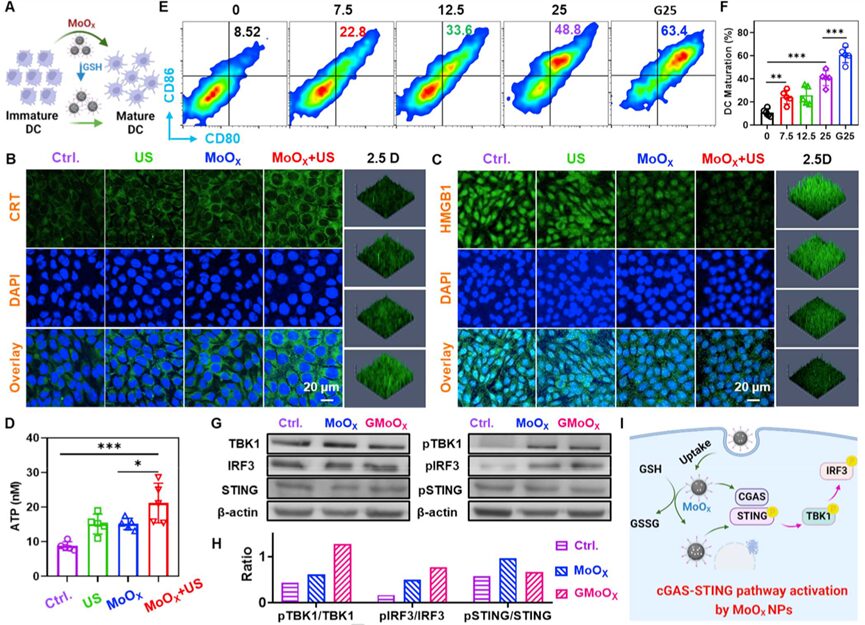 Figure 4. In vitro evaluation of SDT-induced ICD and MoOX-PEG induced metalloimmunotherapy
Utilizing the high cell-killing efficiency and immune effects induced by MoOX-based SDT, the researchers investigated the therapeutic effects of MoOX-PEG combined with US irradiation on 4T1 tumor models. Initially, imaging guidance was required to achieve more precise SDT under US irradiation. Therefore, the DIR labeled MoOX-PEG was intravenously injected into 4T1 tumor-bearing mice (Figure 5A), and it was found that as time progressed, the fluorescence signal of the tumor gradually increased, indicating that MoOX -PEG NPs had high accumulation in the tumor (Figure 5B-C). In vivo imaging 24 hours after intravenous injection further verified the superior tumor accumulation ability of MoOX-PEG (Figure 5D-E). The researchers then investigated the in vivo antitumor effects of MoOX-PEG combined with US irradiation. Twenty-four hours after intravenous injection, these tumors were irradiated under US for 15 minutes, and this process was repeated three times (Figure 5F). Compared to the simple US group and the simple MoOX-PEG group, the SDT group (MoOX-PEG + US) exhibited significant delays in tumor growth, revealing the feasibility of US-enhanced MoOX-PEG based SDT (Figure 5G). Additionally, the survival time of mice in the MoOX-PEG combined with US group was also prolonged compared to the other three control groups (Figure 5I). Meanwhile, hematoxylin and eosin (H&E), TdT mediated dUTP nick end labeling (TdT mediated dUTP nick end labeling, TUNEL), and Ki67 staining results further indicated that the MoOX-PEG combined with US group had the most cell death (Figure 5J), further demonstrating the excellent effects of MoOX induced SDT.
Figure 4. In vitro evaluation of SDT-induced ICD and MoOX-PEG induced metalloimmunotherapy
Utilizing the high cell-killing efficiency and immune effects induced by MoOX-based SDT, the researchers investigated the therapeutic effects of MoOX-PEG combined with US irradiation on 4T1 tumor models. Initially, imaging guidance was required to achieve more precise SDT under US irradiation. Therefore, the DIR labeled MoOX-PEG was intravenously injected into 4T1 tumor-bearing mice (Figure 5A), and it was found that as time progressed, the fluorescence signal of the tumor gradually increased, indicating that MoOX -PEG NPs had high accumulation in the tumor (Figure 5B-C). In vivo imaging 24 hours after intravenous injection further verified the superior tumor accumulation ability of MoOX-PEG (Figure 5D-E). The researchers then investigated the in vivo antitumor effects of MoOX-PEG combined with US irradiation. Twenty-four hours after intravenous injection, these tumors were irradiated under US for 15 minutes, and this process was repeated three times (Figure 5F). Compared to the simple US group and the simple MoOX-PEG group, the SDT group (MoOX-PEG + US) exhibited significant delays in tumor growth, revealing the feasibility of US-enhanced MoOX-PEG based SDT (Figure 5G). Additionally, the survival time of mice in the MoOX-PEG combined with US group was also prolonged compared to the other three control groups (Figure 5I). Meanwhile, hematoxylin and eosin (H&E), TdT mediated dUTP nick end labeling (TdT mediated dUTP nick end labeling, TUNEL), and Ki67 staining results further indicated that the MoOX-PEG combined with US group had the most cell death (Figure 5J), further demonstrating the excellent effects of MoOX induced SDT.
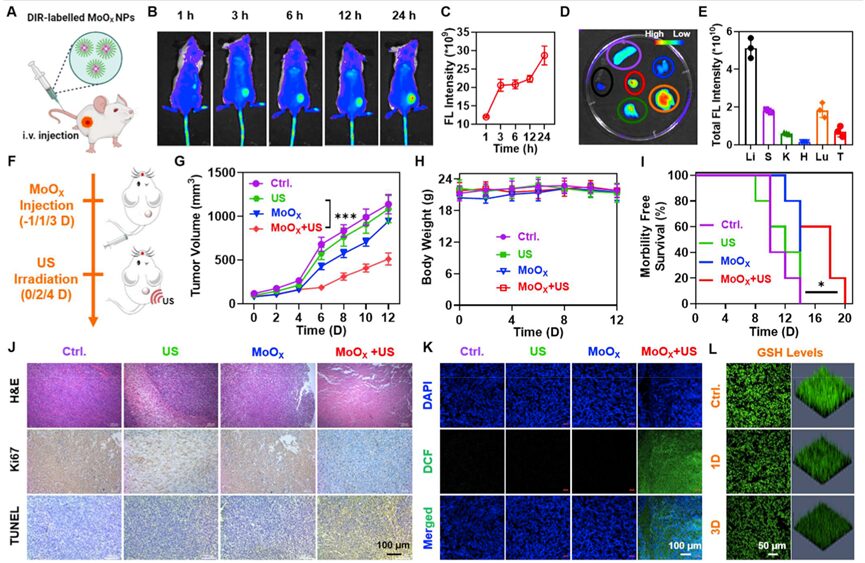 Figure 5. In vivo SDT of MoOX-PEG NPs
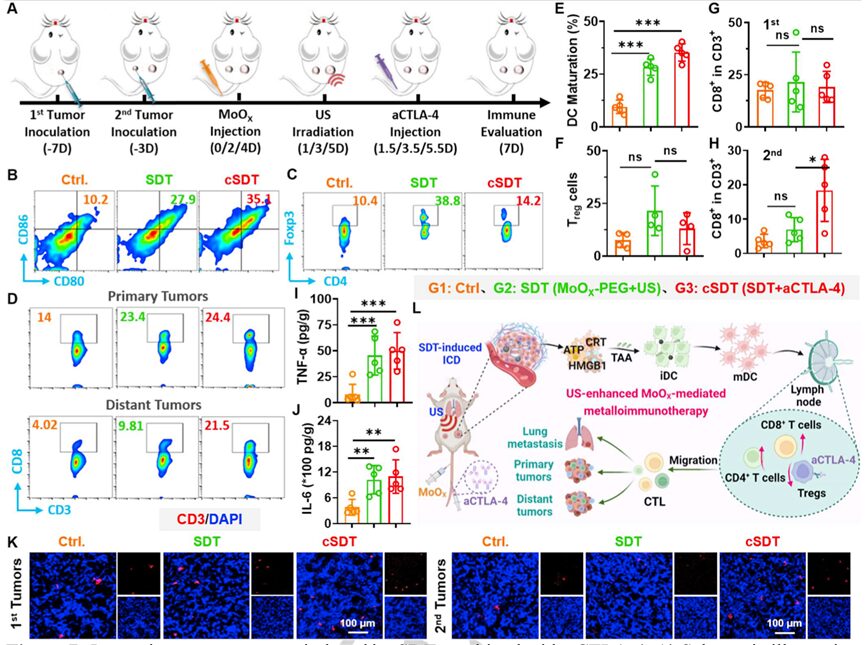
Preclinical studies have shown that ROS-mediated SDT can induce ICD, and then apoptotic or necrotic cell debris releases TAAs, triggering tumor-specific immune responses. Furthermore, cytotoxic T lymphocyte-associated antigen 4 (cytotoxic T lymphocyte-associated antigen 4, CTLA4) is an important negative regulator of immune responses. Blocking its activity against immunosuppressive Tregs with anti-CTLA4 antibodies (aCTLA-4) checkpoint inhibitors has been used as a clinical approach for antitumor immunotherapy. Since the synthesized MoOX-PEG NPs stimulated robust ICD induced by SDT and efficient DC maturation, we investigated the therapeutic effects of MoOX triggered SDT combined with aCTLA-4 treatment to enhance antitumor therapy and immunotherapy. As expected, compared to the use of SDT alone, MoOX triggered SDT + aCTLA-4 (cSDT) showed higher therapeutic outcomes in defeating primary tumors (Figure 6B), and 3/5 of the primary tumors completely disappeared in the cSDT group (Figure 6C). For distant tumors, we found that SDT treatment could partially inhibit the growth of distant tumors. However, cSDT treatment significantly enhanced the inhibitory effect on tumor growth at a distance (Figure 6D-E), indicating that SDT combined with immunotherapy has excellent therapeutic effects, and the survival period of these mice was extended by 2 times (Figure 6F). Lung metastasis experiments in 4T1 tumor-bearing mice further evaluated the therapeutic effects of MoOX triggered SDT combined with aCTLA-4. The SDT group and cSDT group had fewer lung metastases, especially in the cSDT group, while significant cancer metastatic foci were observed in the control group and the group treated with aCTLA-4 alone (Figure 6G-H). Statistical results also showed that compared to the control group, the average lung metastatic nodules in the SDT group and cSDT group were significantly reduced (Figure 6I). SDT combined with immunotherapy effectively inhibits tumor growth and metastasis.
Figure 5. In vivo SDT of MoOX-PEG NPs
Preclinical studies have shown that ROS-mediated SDT can induce ICD, and then apoptotic or necrotic cell debris releases TAAs, triggering tumor-specific immune responses. Furthermore, cytotoxic T lymphocyte-associated antigen 4 (cytotoxic T lymphocyte-associated antigen 4, CTLA4) is an important negative regulator of immune responses. Blocking its activity against immunosuppressive Tregs with anti-CTLA4 antibodies (aCTLA-4) checkpoint inhibitors has been used as a clinical approach for antitumor immunotherapy. Since the synthesized MoOX-PEG NPs stimulated robust ICD induced by SDT and efficient DC maturation, we investigated the therapeutic effects of MoOX triggered SDT combined with aCTLA-4 treatment to enhance antitumor therapy and immunotherapy. As expected, compared to the use of SDT alone, MoOX triggered SDT + aCTLA-4 (cSDT) showed higher therapeutic outcomes in defeating primary tumors (Figure 6B), and 3/5 of the primary tumors completely disappeared in the cSDT group (Figure 6C). For distant tumors, we found that SDT treatment could partially inhibit the growth of distant tumors. However, cSDT treatment significantly enhanced the inhibitory effect on tumor growth at a distance (Figure 6D-E), indicating that SDT combined with immunotherapy has excellent therapeutic effects, and the survival period of these mice was extended by 2 times (Figure 6F). Lung metastasis experiments in 4T1 tumor-bearing mice further evaluated the therapeutic effects of MoOX triggered SDT combined with aCTLA-4. The SDT group and cSDT group had fewer lung metastases, especially in the cSDT group, while significant cancer metastatic foci were observed in the control group and the group treated with aCTLA-4 alone (Figure 6G-H). Statistical results also showed that compared to the control group, the average lung metastatic nodules in the SDT group and cSDT group were significantly reduced (Figure 6I). SDT combined with immunotherapy effectively inhibits tumor growth and metastasis.
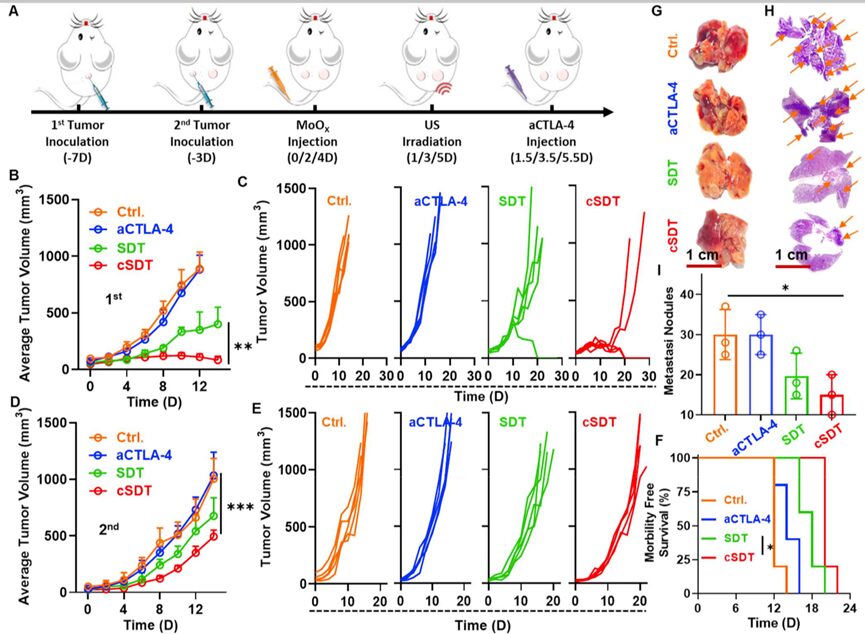 Figure 6. MoOX induced sonodynamic immunotherapy combined with aCTLA-4
To elucidate the therapeutic mechanism of SDT combined with aCTLA-4 blockade, the researchers studied various immune responses in primary and distant tumors after treatment (Figure 7A). By analyzing the single-cell suspensions of lymph nodes and tumors from mice treated with different therapies, it was found that compared to untreated mice, the SDT and cSDT groups injected with MoOX-PEG significantly promoted the maturation of DCs (Figure 7B-E), which would be beneficial for subsequent antigen presentation. Additionally, in mice treated with SDT and cSDT, the number of mature DCs significantly increased, demonstrating high levels of release of various cytokines such as tumor necrosis factor α (tumor necrosis factor α, TNF-α), interleukin-6 (interleukin-6, IL-6), and interleukin-1β (interleukin- 1β, IL-1β) to modulate the immune system and trigger systemic antitumor immunity (Figure 7I-J).
Figure 7. In vivo immune responses induced by SDT combined with aCTLA-4
Ultra-small oxygen-deficient MoOX NPs were used as novel sonosensitizers and stimulators for sonodynamic immunotherapy of cancer. Due to the oxygen vacancy structure of MoOX-PEG, it exhibited effective ROS generation performance after surface modification, thereby amplifying tumor oxidative stress. Additionally, MoOX-PEG NPs can deplete GSH, breaking the redox balance, weakening the tumor’s antioxidant capacity, and further increasing the ROS levels during the SDT process. Interestingly, the increased oxygen defect structure hindered the recombination of electron-hole pairs, and MoOX-PEG NPs exhibited higher generation efficiency after consuming a large amount of GSH. Meanwhile, MoOX-PEG NPs themselves can further stimulate DC maturation and activate the cGAS-STING pathway. Under US irradiation, MoOX-PEG generates a large amount of ROS, inducing high cell-killing efficiency and strong ICD effects, effectively inhibiting tumor growth. Furthermore, due to the strong ICD induced by SDT and the efficient DC maturation induced by MoOX-PEG, combining MoOX triggered SDT with aCTLA-4 combination therapy will further enhance antitumor treatment, inhibit tumor metastasis, trigger immune responses, and effectively suppress tumor growth. Finally, the long-term toxicity of MoOX-PEG NPs to mice can be neglected. Therefore, this work emphasizes a promising strategy for integrating GSH-enhanced SDT and metalloimmunotherapy for effective cancer treatment.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202215467
Source: BioMed Technology Note: This only represents the author’s personal views. The author’s level is limited, and if there are any scientific inaccuracies, please leave a comment below to correct!
Figure 6. MoOX induced sonodynamic immunotherapy combined with aCTLA-4
To elucidate the therapeutic mechanism of SDT combined with aCTLA-4 blockade, the researchers studied various immune responses in primary and distant tumors after treatment (Figure 7A). By analyzing the single-cell suspensions of lymph nodes and tumors from mice treated with different therapies, it was found that compared to untreated mice, the SDT and cSDT groups injected with MoOX-PEG significantly promoted the maturation of DCs (Figure 7B-E), which would be beneficial for subsequent antigen presentation. Additionally, in mice treated with SDT and cSDT, the number of mature DCs significantly increased, demonstrating high levels of release of various cytokines such as tumor necrosis factor α (tumor necrosis factor α, TNF-α), interleukin-6 (interleukin-6, IL-6), and interleukin-1β (interleukin- 1β, IL-1β) to modulate the immune system and trigger systemic antitumor immunity (Figure 7I-J).
Figure 7. In vivo immune responses induced by SDT combined with aCTLA-4
Ultra-small oxygen-deficient MoOX NPs were used as novel sonosensitizers and stimulators for sonodynamic immunotherapy of cancer. Due to the oxygen vacancy structure of MoOX-PEG, it exhibited effective ROS generation performance after surface modification, thereby amplifying tumor oxidative stress. Additionally, MoOX-PEG NPs can deplete GSH, breaking the redox balance, weakening the tumor’s antioxidant capacity, and further increasing the ROS levels during the SDT process. Interestingly, the increased oxygen defect structure hindered the recombination of electron-hole pairs, and MoOX-PEG NPs exhibited higher generation efficiency after consuming a large amount of GSH. Meanwhile, MoOX-PEG NPs themselves can further stimulate DC maturation and activate the cGAS-STING pathway. Under US irradiation, MoOX-PEG generates a large amount of ROS, inducing high cell-killing efficiency and strong ICD effects, effectively inhibiting tumor growth. Furthermore, due to the strong ICD induced by SDT and the efficient DC maturation induced by MoOX-PEG, combining MoOX triggered SDT with aCTLA-4 combination therapy will further enhance antitumor treatment, inhibit tumor metastasis, trigger immune responses, and effectively suppress tumor growth. Finally, the long-term toxicity of MoOX-PEG NPs to mice can be neglected. Therefore, this work emphasizes a promising strategy for integrating GSH-enhanced SDT and metalloimmunotherapy for effective cancer treatment.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202215467
Source: BioMed Technology Note: This only represents the author’s personal views. The author’s level is limited, and if there are any scientific inaccuracies, please leave a comment below to correct!