Expert Commentary


Professor Feng Jifeng
Chief Physician, Professor, Doctoral Supervisor
Secretary of the Party Committee, Jiangsu Provincial Cancer Hospital/Nanjing Medical University Affiliated Cancer Hospital
Member of the Expert Committee on Rational Drug Use, National Health Commission
Council Member of the Chinese Medical Association, Member of the Internal Medicine Group of the Oncology Branch
Vice President of the Oncology Branch of the Chinese Physician Association
Executive Director of the Chinese Anti-Cancer Association, Chinese Society of Clinical Oncology
Chairman of the Clinical Chemotherapy Professional Committee of the Chinese Anti-Cancer Association
Vice Chairman of the Lymphoma Committee and Member of the Breast Cancer Committee of the Chinese Anti-Cancer Association
Vice Chairman of the Rare Disease Committee and Standing Committee Member of the Breast Cancer Committee of the Chinese Society of Clinical Oncology
Chairman of the Oncology Branch of Jiangsu Provincial Geriatric Medicine Association
Chairman of the Chemotherapy Committee and Lymphoma Committee of Jiangsu Provincial Anti-Cancer Association
Incoming Chairman of the Oncology Chemotherapy and Biological Therapy Branch of Jiangsu Provincial Medical Association
Chairman of the Oncology Branch of Jiangsu Provincial Preventive Medicine Association
From a global perspective, cancer remains a serious threat to public health, with breast cancer being the most prevalent malignancy among women in China. It is hoped that through the continuous exploration of new medical technologies and treatment concepts by research institutions and clinical physicians, we can further improve the clinical cancer prevention and treatment system in China, implement the Healthy China 2030 guidelines, and accelerate the achievement of the goal of increasing the 5-year survival rate of cancer patients in China to 46.6%, allowing more Chinese patients to access new drugs and enjoy better quality of life.
This case involves a HER2-positive breast cancer patient whose treatment options were extremely limited after progression following dual-targeted therapy and TKI treatment. After the application of Vadiscituzumab, good tumor control was achieved. It is hoped that Vadiscituzumab will provide safe and effective personalized treatment for more patients with HER2-expressing breast cancer, and that more clinical studies tailored to national conditions will be conducted in the future, with the support of national policies, to help more cancer patients live longer and better.

Author:Chief Physician Qian Zhiying, Jiangsu Provincial Cancer Hospital

Professor Qian Zhiying
Chief Physician, Jiangsu Provincial Cancer Hospital, Master’s Supervisor
Member of the National Natural Science Foundation Review Expert Database
Committee Member of the Breast Cancer Group of Jiangsu Provincial Anti-Cancer Association
Expert in the internal treatment of various common malignant tumors. Has extensive experience in the diagnosis and treatment of various malignant tumors, biological treatment, targeted therapy, as well as acute conditions and complications of tumors, especially proficient in the diagnosis and treatment of breast cancer, liver cancer, and esophageal cancer.
Has hosted and led three provincial-level research projects, contributed to five monographs including “Comprehensive Treatment of Breast Cancer”, and has undertaken clinical research work on new anti-cancer drugs multiple times.
Awarded the First Prize for Scientific and Technological Progress in Jiangsu Province and the Nanjing Municipal Scientific and Technological Progress Award.

Case Introduction

1Basic Information:
Case Data: Xia, Female, 64 years old
Case Provider: Jiangsu Provincial Cancer Hospital, Qian Zhiying
Disease Diagnosis: Advanced Metastatic Breast Cancer
2Medical History:
2020-05-12 Left breast mass discovered, biopsy pathology indicated invasive carcinoma. Immunohistochemistry: ER (occasional cells +, <1% 1+), PR (-), Her-2 (3+), Ki67 (30%+). FISH test results indicated HER-2 gene amplification. Whole body bone imaging suggested multiple bone metastases. Staging IV.
2020-05-26 underwent PHT chemotherapy regimen for 6 cycles. Specific medication included “liposomal paclitaxel 210mg + trastuzumab initial 462mg, subsequent 352mg + pertuzumab initial 840mg, subsequent 420mg”, along with zoledronic acid for bone modification treatment.
2020-09 follow-up at an external hospital indicated an increase in the size of the left breast mass and non-mass-like enhancement, with new bone metastases, with disease progression (PD) and progression-free survival (PFS) of 4 months.
2020-09-25 underwent “modified radical mastectomy for left breast cancer”. Postoperative pathology indicated a maximum tumor diameter of 3.5cm, other dimensions of 3cmx2.5cm, histological type of invasive ductal carcinoma, non-special type. Axillary lymph nodes 20 pieces; multiple vascular invasions present. Immunohistochemistry indicated: ER (20%+), PR (-), Her-2 (3+), Ki67 (90%+), AR (>90%+), CK5/6 (-). Postoperative diagnosis was T2N3aM1 stage.
2020-10-01~2021-09-01 treated with oral pyrotinib + capecitabine, PFS 11 months.
2021-08-30 follow-up CT at an external hospital indicated liver and adrenal metastases, with progression of bone metastases.
2021-09-17 transferred to Jiangsu Provincial Cancer Hospital. Physical examination: left breast absent, postoperative changes on the left chest wall, no abnormalities in the right breast. Liver hard, slight tenderness, 3cm below the right costal margin. ECOG score 1. Admission diagnosis was (left) breast malignant tumor, invasive ductal carcinoma (TNM:T2N3aM1 IV stage), HER-2 overexpression type. After admission, the patient developed jaundice and abnormal liver function indicators.
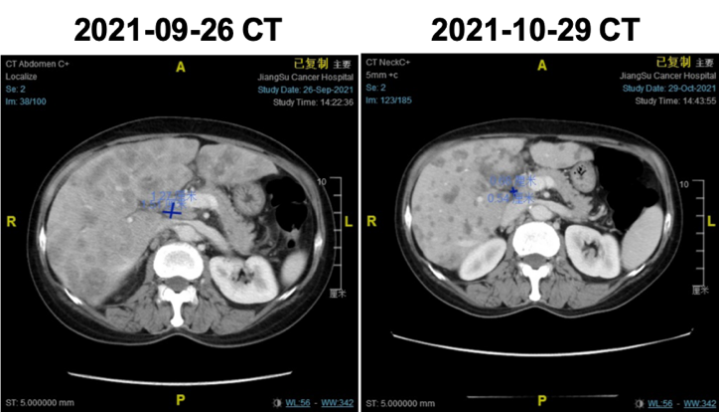
2021-09-26 CT indicated: diffuse liver metastases, possible adrenal nodular metastases on the left side.
3Treatment Process:
On 2021-09-28, treatment with Vadiscituzumab 60mg weekly dosage was started for 4 times, along with hepatoprotective treatment. After four treatments, the patient developed grade 3 rash, accompanied by skin itching, which subsided after taking antihistamines.
3Treatment Results:
2021-10-29 follow-up indicated significant reduction in liver lesions. Jaundice subsided, and liver function improved significantly. Efficacy evaluation was PR.
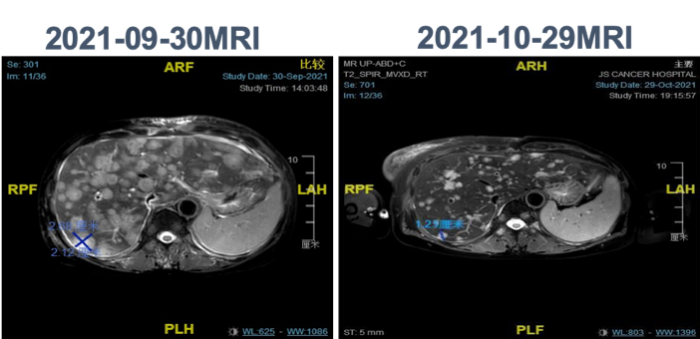
Significant reduction in liver lesions
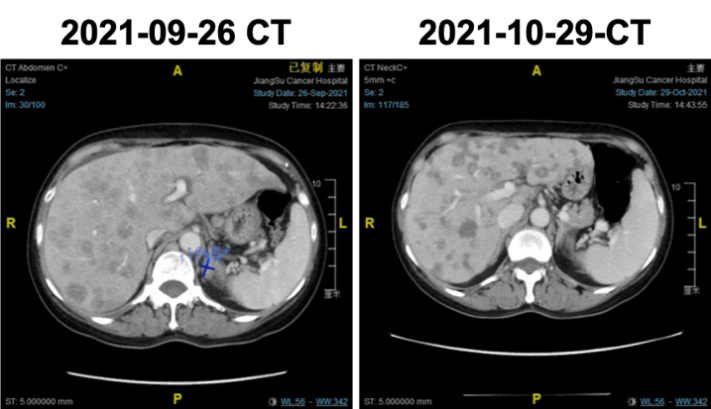
Adrenal imaging assessment
Hilar lymph node imaging assessment
Changes in biochemical indicators
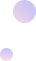
Case Summary

The patient was initially diagnosed with advanced breast cancer with multiple bone metastases. After undergoing 6 cycles of dual-target systemic therapy at an external hospital, the disease progressed with a PFS of 4 months. Therefore, a modified radical surgery was performed for the local lesions, followed by oral pyrotinib + capecitabine treatment with a PFS of 11 months. Subsequent follow-up revealed multiple metastases in lymph nodes, liver, and adrenal glands. After transferring to our hospital and with comprehensive assessment and informed consent from the patient, Vadiscituzumab weekly dosage treatment was administered, along with hepatoprotective treatment for jaundice. After four treatments, follow-up indicated significant reduction in liver lesions, jaundice subsided, and liver function improved significantly. The patient experienced grade 3 rash during treatment, which subsided after taking antihistamines, with no other adverse reactions. Overall, this patient had a rapid disease progression, and after failure of first-line dual-target therapy and progression after second-line pyrotinib, the application of Vadiscituzumab achieved satisfactory efficacy.
Antibody-drug conjugate (ADC) drugs are composed of monoclonal antibodies, linkers, and small molecule cytotoxic drugs, combining the targeting ability of antibodies with the killing power of small molecule drugs, enabling precise targeting of tumor cells.
Vadiscituzumab, as a Class 1 innovative original HER2-ADC in China, utilizes the cytotoxic drug MMAE (monomethyl auristatin E), significantly achieving rapid tumor shrinkage and downstaging (TTR=2~3 cycles) for high-proliferation and high-burden tumors. The American Society of Clinical Oncology (ASCO) 2021 conference presented a summary analysis of two studies on Vadiscituzumab for HER2-positive and HER2-low expressing advanced or metastatic breast cancer. Data showed that Vadiscituzumab exhibited excellent efficacy in both HER2-positive and HER2-low expressing breast cancer patients, with objective response rates (ORR) of 42.9% and 39.6%, respectively, and median progression-free survival (PFS) of 5.7 months, with disease control reaching 90%.
Preliminary mid-term data from Phase I of the C006 study indicated that for HER2-positive breast cancer patients with liver metastases, the Vadiscituzumab group achieved an ORR of 63.2% and a median PFS of 12.5 months. Vadiscituzumab has recently received breakthrough therapy designation from the NMPA, targeting patients with HER2-positive liver metastatic breast cancer.
This case involves a HER2-positive breast cancer patient, but treatment options after failure of dual-target therapy were extremely limited. After the application of Vadiscituzumab, good tumor control was achieved. Vadiscituzumab, with its precision, potency, and safety, has become an ideal treatment option for HER2-expressing breast cancer, aiming to provide clinical practice choices for more patients urgently needing anti-HER2 treatment and to offer safer personalized drug treatment options for more cancer patients.
(Note from the case provider: Given the limited treatment options available, the above plan was provided to the patient, and full communication was conducted with the patient. The purpose of sharing this case is to provide a diagnostic and treatment approach for clinical doctors encountering similar refractory patients, for discussion.)