iNature
In clinical practice, capsules are widely used for oral administration of various drugs, but real-time tracking of drug release behavior and adjusting dosage based on therapeutic effects remains challenging.
On December 11, 2024, Song Jibin from Beijing University of Chemical Technology and Mu Jing from Peking University co-authored a research paper titled “NIR-II Ratiometric Optical Theranostic Capsule for In Situ Diagnosis and Precise Therapy of Intestinal Inflammation” published online in ACS Nano.
The study developed a theranostic capsule loaded with two types of fluorescent nanoparticles, H2O2-responsive Janus Ag/Ag2S nanoparticles (Ag/Ag2SJNPs) and down-conversion nanoparticles (DCNP), along with the drug dexamethasone (Dex).
Ag/Ag2SJNPs exhibit high sensitivity fluorescence (FL) signal at 1250nm, while the FL signal from DCNP remains stable at 1550nm under physiological conditions. The ratio of these two FL signals forms a ratiometric FL signal that correlates with H2O2 concentration, with a detection limit of 1.7μM. Moreover, the capsule can be accurately delivered to the intestine, simultaneously releasing JNPs and DCNPs. The H2O2-triggered ratiometric fluorescence signal and imaging can diagnose inflammation and indicate its location. At the same time, the encapsulated Dex is released in the diseased area, allowing for real-time tracking of therapeutic effects through ratiometric imaging and providing guidance. The theranostic capsule system offers a method for quantitative detection of disease biomarkers and localized release of therapeutic agents, avoiding overdose and reducing side effects.

Inflammatory diseases such as autoimmune diseases and infectious diseases are among the major threats to global human health. Inflammation can be diagnosed through in vitro methods such as routine blood tests, stool tests, or tissue biopsies, which are time-consuming and lack precision. In contrast, in situ diagnostic probes offer a more direct method for detecting inflammation. Recent studies have used MOF-based fluorescent (FL) nanoparticles to diagnose pneumonia and metal-based photoacoustic nanoparticles to detect arthritis. However, traditional imaging methods are primarily qualitative and struggle to accurately quantify complex inflammatory microenvironments. Therefore, there is an urgent need to develop advanced technologies and nanoparticles capable of quantifying inflammation in situ.
Generally, most inflammatory diagnostic probes are locally injected into the site of inflammation, such as in local arthritis. However, for intestinal inflammation, oral administration is simple, safe, cost-effective, and the oral dosage form is easy to mass-produce, making it a preferred method. Traditional small-molecule probes often lack stability, especially in terms of pH sensitivity, and are easily degraded by gastric acid and digestive juices after oral administration, limiting their application in oral delivery. Therefore, there is a need to develop stable probes suitable for oral delivery. Only a few oral probes can combine diagnostic imaging of the inflammation site with real-time therapeutic guidance. To address the issue of gastric degradation, probes can be loaded into a pH-sensitive sustained-release coating that can withstand harsh acidic gastric environments, ensuring gradual degradation under intestinal pH conditions. In this way, oral probes can not only detect inflammation but also monitor the treatment process of inflammation, achieving integrated diagnosis and treatment. Inorganic nanoparticles show great potential in the development of oral theranostic probes due to their optical stability and ease of surface adaptability.
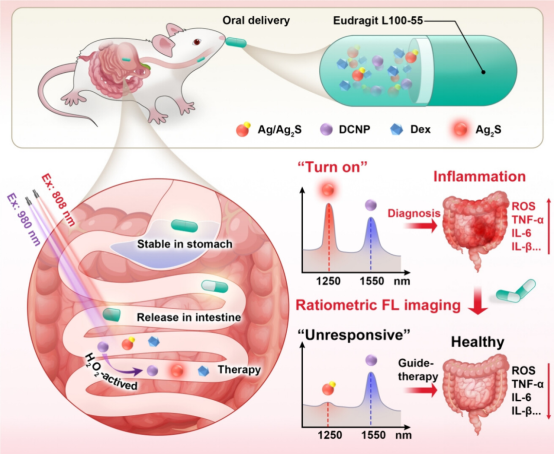
Figure 1 Schematic diagram of the preparation of ratiometric fluorescent nanoparticles for in situ diagnosis and precise treatment of inflammation (excerpted from ACS Nano)
Inflammatory bowel disease (IBD) is often caused by the immune system attacking the tissues of the digestive system, often leading to recurrence after treatment.During intestinal inflammation, macrophages polarize from M2 to M1 phenotype, significantly increasing the production of pro-inflammatory cytokines such as IL-6, TNF-α, IL-1β, IL-12, while the expression of anti-inflammatory cytokine IL-10 decreases. These changes lead to increased reactive oxygen species generation, exacerbating oxidative stress, leading to cell apoptosis, and further activating the inflammatory response. Hydrogen peroxide (H2O2) is a key reactive oxygen species in inflammation and a critical signaling molecule reflecting the severity of inflammation. Compared to normal tissue, inflamed tissue often has higher levels of H2O2. Furthermore, endogenous bioenergy can produce H2O2 at the site of inflammation. Therefore, intestinal H2O2 can serve as a biomarker for diagnosing IBD. Given the significant potential of H2O2 as an internal reaction stimulus, developing H2O2-responsive nanomaterials for in situ quantitative detection of H2O2 can not only locate the inflammation area but also improve the therapeutic efficiency of inflammation. However, clinical blood testing methods have low sensitivity and cannot identify the inflammation area, making it difficult to measure in situ H2O2 levels. Therefore, there is an urgent need to develop accurate, sensitive, and quantitative techniques to monitor in situ H2O2 levels in real-time while achieving targeted therapy for inflammation.
IBD is usually treated with potent anti-inflammatory drugs (such as prednisone), which help alleviate symptoms such as abdominal pain, vomiting, diarrhea, and rectal bleeding. Although symptom relief indicates that inflammation has subsided, residual inflammation in the intestine still exists, which may lead to IBD recurrence. This recurrence can even cause severe complications and increase the risk of cancer. Diagnosis of IBD usually requires invasive and uncomfortable endoscopy, necessitating a high level of clinical expertise. In contrast, the near-infrared (NIR)-II window (1000–1700nm) has more significant advantages than NIR-I (650–900nm), such as reduced light scattering and autofluorescence, thereby enhancing imaging of inflamed areas, with lower background signal interference, higher sensitivity, higher resolution, and deeper tissue penetration. Therefore, developing sensitive and reliable NIR-II FL nanoparticles is crucial for in vivo FL imaging and detection of bioactive molecules in situ. In this regard, ratiometric FL imaging can mitigate background signal interference from factors such as nanoprobe concentration and laser intensity, providing more accurate and sensitive detection compared to traditional absolute intensity imaging methods.
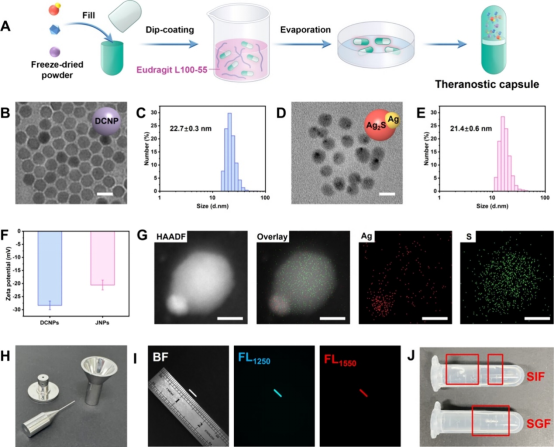
Figure 2 Preparation and characterization of intestinal capsules (excerpted from ACS Nano)
The study developed a ratiometric optical capsule for in situ real-time diagnosis and monitoring of IBD treatment. H2O2-activatable nanoprobe and model therapeutic agents were encapsulated in a microparticle coated with intestinal polymers for oral delivery. Specifically, Ag/Ag2S Janus nanoparticles (JNP), down-conversion nanoparticles (DCNP), and dexamethasone (Dex) were encapsulated in the capsule, with the outermost layer coated with Eudragit L100-55 (a copolymer of methacrylic acid and methyl methacrylate). Eudragit L100-55 is a pH-responsive polymer commonly used for sustained drug release in the intestine (5.5-7.5). The developed capsule measures 8.4mm in length and 1.27mm in diameter, suitable for oral administration in mice. To increase biocompatibility, PEG was used to modify Ag/Ag2S Janus nanoparticles and DCNPs. As the capsule gradually dissolves in the intestinal area, the effective payload is gradually released, allowing Dex to exert its therapeutic effect. The corresponding released Ag/Ag2S and DCNPs nanoprobe can respond to the intestinal in situ biomarker H2O2 to detect IBD. Ag is partially etched by intestinal H2O2, restoring the Ag2S FL signal at 1250nm (FL1250). In contrast, DCNPs are insensitive to (FL1250), providing a standard reference signal at 1550nm (FL1550), enabling in situ H2O2 NIR-II ratiometric FL imaging of the inflamed area. Quantitative detection of in vivo H2O2 can not only accurately identify the inflammation area but also track the treatment process and provide real-time therapeutic guidance, thereby reducing side effects and preventing inflammation recurrence.
References:
https://pubs.acs.org/doi/10.1021/acsnano.4c12894

—END—
This content is original from 【iNature】,
Please indicate the source as 【iNature】
Add WeChat Group
iNature gathers 40,000 life science researchers and doctors. We have formed 80 comprehensive groups (16 PI groups and 64 doctoral groups), and also established specialized groups in related fields (plants, immunology, cells, microbiology, gene editing, neurology, chemistry, physics, cardiovascular, oncology, etc.).Note: Please indicate when joining the group (format: school + major + name; if you are a PI/professor, pleaseindicate as PI/professor, otherwise, it will be assumed you are a doctoral student, thank you).You can first add the editor’s WeChat ID (love_iNature), or long press the QR code to add the editor, and then join the relevant group. Please refrain from unnecessary inquiries.


Submission, collaboration, and reprint authorization matters
Please contact WeChat ID:13701829856 or email:[email protected]
If you find this article appealing, please click here!