Skip to content
Recently, Professor Koen Raemdonck’s team at the University of Ghent, Belgium published a research paper titled “A lipid nanoparticle platform for mRNA delivery through repurposing of cationic amphiphilic drugs” in The Journal of Controlled Release, Volume 350, 2022.
The team utilized various cationic amphiphilic drugs as structural and functional components to prepare lipid nanoparticles (LNPs) for RNA delivery, exploring how different types of cationic amphiphilic drugs affect cellular uptake and translation of mRNA within LNPs. They confirmed that the prepared LNPs-mRNA could successfully transfect bovine corneal epithelial cells, and further in vivo experiments showed that it also has potential for mRNA delivery in rabbit corneal epithelial cells, thus proposing a universal drug combination delivery system.
Nucleic acid therapy, as an emerging treatment technology, has been applied to various diseases, with its therapeutic effect highly dependent on the delivery system. Among them, LNPs are currently the preferred carrier material for RNA delivery, overcoming many extracellular and intracellular barriers in in vivo administration. Cationic amphiphilic drugs are a class of small molecule drugs with amphiphilic and weakly basic properties, which include antidepressants, antihistamines, and antihypertensives.
The team previously confirmed that cationic amphiphilic drugs can be used to stimulate lysosomal destabilization in cancer cells, thereby improving siRNA delivery. This article indicates that cationic amphiphilic drugs can also enhance mRNA delivery as structural and functional components of LNPs. The authors referred to the formulation using cationic amphiphilic drugs as structural components for mRNA delivery in LNPs as CADosomes, confirming that cationic amphiphilic drugs still possess pharmacological activity in the combined drug delivery system CADosomes.
Moreover, the researchers confirmed that the structure of cationic amphiphilic drugs affects LNP formation and cellular uptake. This study utilized primary bovine corneal epithelial cells instead of the more easily transfected HeLa cells, confirming the high mRNA delivery efficiency of CADosomes, which are particularly suitable for local administration in corneal repair.
This study prepared LNPs using eight different cationic amphiphilic drugs in combination with DOPE, further complexing mRNA onto their surface. The five cationic amphiphilic drugs that successfully formed LNPs are Nortriptyline hydrochloride (NT), Amitriptyline hydrochloride (AMI), Doxepin hydrochloride (DSI), Imipramine hydrochloride (IMI), and Desloratadine (DES).
With a positively charged headgroup, NT can interact with anionic phosphatidylserine membranes, while the hydrophobic aromatic ring can insert into the lipid membrane. Stable cationic LNPs can be obtained by simply mixing NT with DOPE (50:50 molar ratio) (as shown in Figure 1).
After complexing with mRNA, the LNPs change to a negative charge, and transmission electron microscopy (TEM) shows a spherical morphology with a dense lipid core. The NT-DOPE CADosome prepared by the ethanol dilution method (ED) with an N/P ratio of 9 was selected as the optimal composition.
To expand applications, other cationic amphiphilic drugs were combined with DOPE and mRNA to prepare CADosomes. Flow cytometry was used to investigate the fluorescence signal generated by Cy5-labeled mRNA and eGFP in HeLa cells, exploring the relationship between uptake and expression—finding that only DSI-DOPE exhibited mRNA internalization efficiency comparable to NT-DOPE.
This trend was also reflected in eGFP expression, where only DSI-DOPE demonstrated mRNA delivery efficiency similar to NT-DOPE, with up to 90% of eGFP+ cells exhibiting high expression levels.
Due to the differences in the structure of cationic amphiphilic drugs, the uptake and expression levels of each LNP vary significantly. Cationic amphiphilic drugs with a secondary amine group and a greater distance between the tricyclic structure are optimal for the formation of CADosomes and intracellular mRNA delivery.
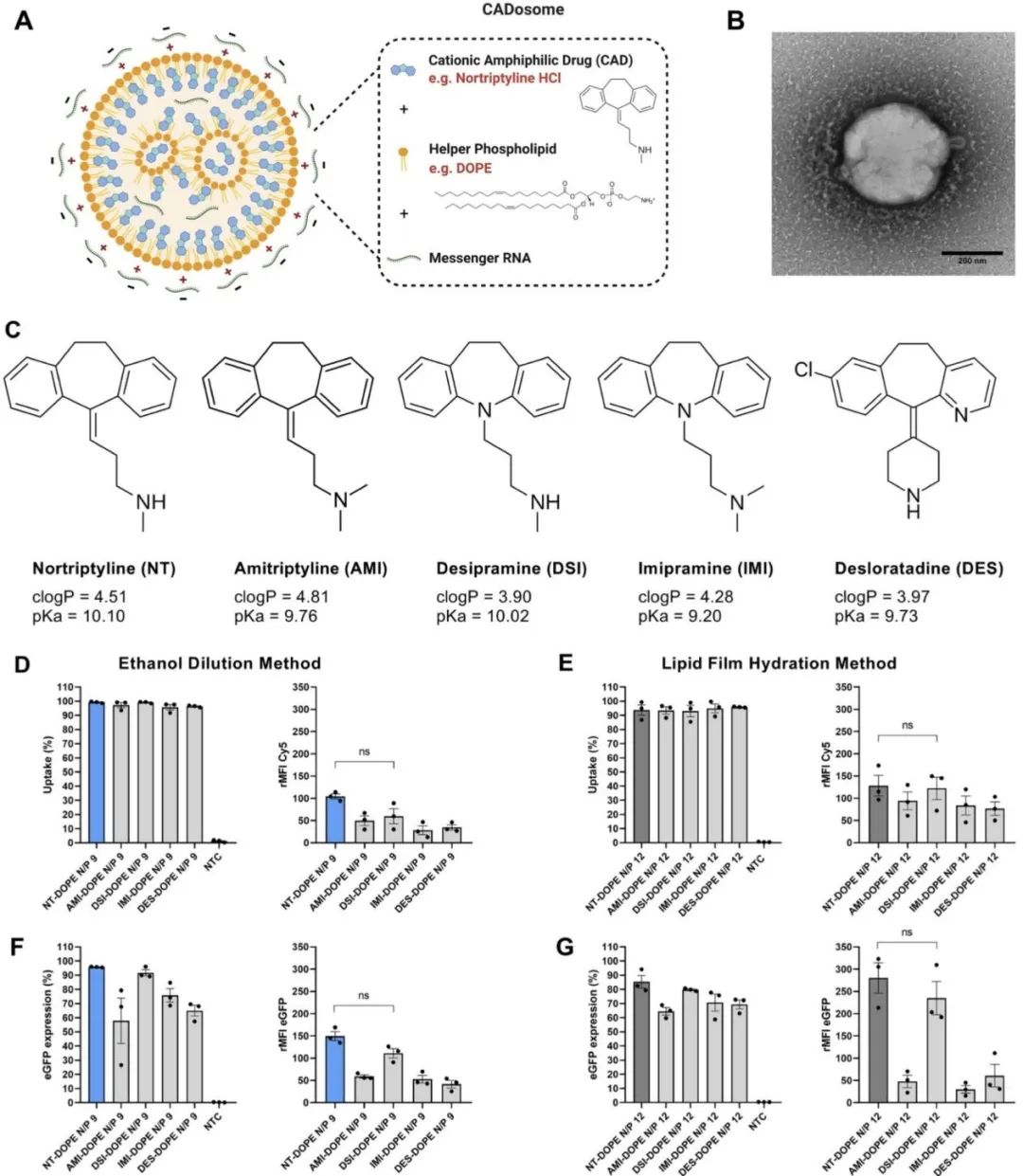 Figure 1. Evaluation of CADosomes delivering mRNA in HeLa cells. (A) Schematic diagram of NT-DOPE mRNA CADosomes obtained by the ethanol dilution method (ED) or lipid film hydration method (LFH). (B) Typical transmission electron microscopy (TEM) image of NT-DOPE CADosomes loaded with enhanced green fluorescent protein encoding messenger RNA (EGFP-mRNA) prepared by ED. The scale bar corresponds to 200 nanometers. (C) Molecular structures of cationic amphiphilic drugs that can bind with DOPE (50:50 molar ratio) to form CADosomes. (D-E) Flow cytometry measuring the cellular uptake of AMI-DOPE, DSI-DOPE, IMI-DOPE, and DES-DOPE, respectively loaded with Cy5-labeled or eGFP-mRNA, compared with NT-DOPE CADosome prepared by the ethanol dilution method (D, F) and lipid film hydration method (E, G), using the optimal N/P ratio.
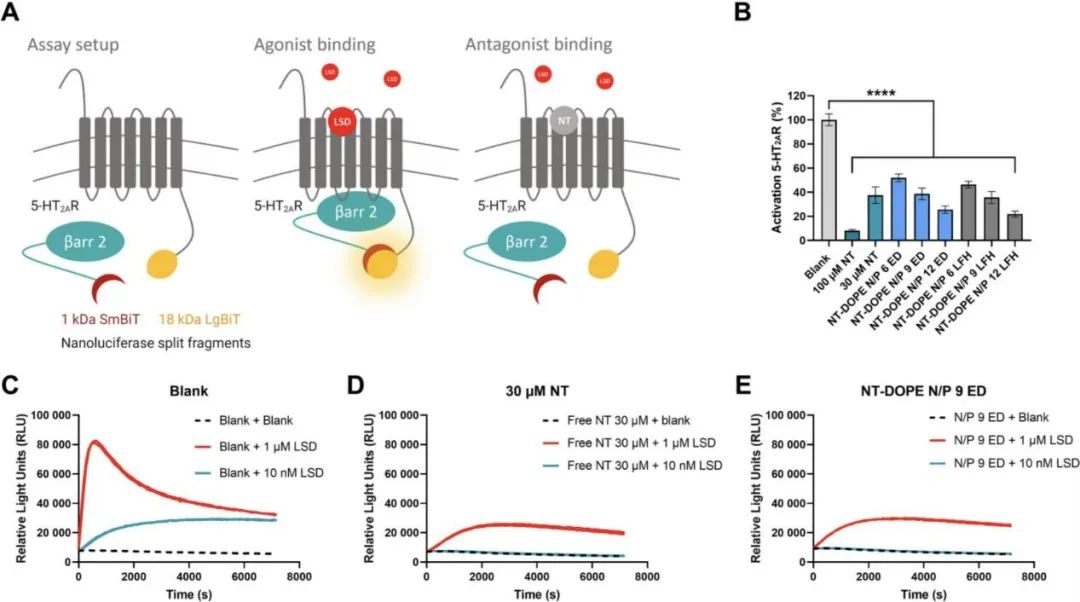
The team confirmed through a dual-luciferase assay that the cationic amphiphilic drugs in the formulation still possess pharmacological activity. HEK293T cells (human embryonic kidney cells) stably express two inactive luciferase cleavage fragments, coupled to 5-HT2AR (5-hydroxytryptamine 2A receptor) and cytoplasmic protein β-Arrestin2 (βArr2) (as shown in Figure 2).
The binding of receptor agonists (such as lysergic acid diethylamide, LSD) leads to the recruitment of βARR2 to 5-HT2AR, causing luciferase complementation, resulting in detectable bioluminescence. The binding of the pharmacologically active receptor antagonist Nortriptyline (NT) inhibits LSD-induced βARR2 recruitment and subsequent luciferase complementation, thereby reducing the intensity of bioluminescence.
As expected, increasing the concentration of free NT (30μM and 100μM) reduced or even completely blocked the activation of 5-HT2AR induced by 1μM and 10nM agonist LSD, respectively.
Notably, as the N/P ratio increases, transfecting HEK293T cells with NT-DOPE CADosome weakens LSD-induced 5-HT2AR activation. There was no significant difference in the antagonistic effect on 5-HT2AR between 30μM free NT and formulation NT-DOPE CADosomes N/P 9. These data indicate that the pharmacological activity of Nortriptyline remains unaffected after forming CADosomes.
Figure 2. Evaluation of the pharmacological activity of Nortriptyline in CADosome formulations using a dual-luciferase assay. (A) Schematic diagram of the dual-luciferase assay. (B) Percentage of 5HT2AR activation induced by blank, free NT (30μM, 100μM), and various NT-DOPE doped eGFP-mRNA loaded CADosomes, measured by calculating the area under the receptor activation curve (AUC) value. 1μM of LSD was added to all samples, and luminescence was monitored continuously for 2 hours. n=3. (C-E) Representative activation spectra for blank, 30μM free NT, and mRNA NT-doped N/P9.
This article also evaluated the high transfection efficiency of CADosomes using HeLa reporter cell lines stably expressing the Cre reporter plasmid pLV-CMV-LoxP-DsRed-LoxP-eGFP and primary bovine corneal epithelial cells (PBCECs). Finally, the safety and efficacy of CADosomes for local nucleic acid delivery to the cornea were demonstrated through eye drop administration in a rabbit eye model (as shown in Figure 3).
Figure 1. Evaluation of CADosomes delivering mRNA in HeLa cells. (A) Schematic diagram of NT-DOPE mRNA CADosomes obtained by the ethanol dilution method (ED) or lipid film hydration method (LFH). (B) Typical transmission electron microscopy (TEM) image of NT-DOPE CADosomes loaded with enhanced green fluorescent protein encoding messenger RNA (EGFP-mRNA) prepared by ED. The scale bar corresponds to 200 nanometers. (C) Molecular structures of cationic amphiphilic drugs that can bind with DOPE (50:50 molar ratio) to form CADosomes. (D-E) Flow cytometry measuring the cellular uptake of AMI-DOPE, DSI-DOPE, IMI-DOPE, and DES-DOPE, respectively loaded with Cy5-labeled or eGFP-mRNA, compared with NT-DOPE CADosome prepared by the ethanol dilution method (D, F) and lipid film hydration method (E, G), using the optimal N/P ratio.
The team confirmed through a dual-luciferase assay that the cationic amphiphilic drugs in the formulation still possess pharmacological activity. HEK293T cells (human embryonic kidney cells) stably express two inactive luciferase cleavage fragments, coupled to 5-HT2AR (5-hydroxytryptamine 2A receptor) and cytoplasmic protein β-Arrestin2 (βArr2) (as shown in Figure 2).
The binding of receptor agonists (such as lysergic acid diethylamide, LSD) leads to the recruitment of βARR2 to 5-HT2AR, causing luciferase complementation, resulting in detectable bioluminescence. The binding of the pharmacologically active receptor antagonist Nortriptyline (NT) inhibits LSD-induced βARR2 recruitment and subsequent luciferase complementation, thereby reducing the intensity of bioluminescence.
As expected, increasing the concentration of free NT (30μM and 100μM) reduced or even completely blocked the activation of 5-HT2AR induced by 1μM and 10nM agonist LSD, respectively.
Notably, as the N/P ratio increases, transfecting HEK293T cells with NT-DOPE CADosome weakens LSD-induced 5-HT2AR activation. There was no significant difference in the antagonistic effect on 5-HT2AR between 30μM free NT and formulation NT-DOPE CADosomes N/P 9. These data indicate that the pharmacological activity of Nortriptyline remains unaffected after forming CADosomes.
Figure 2. Evaluation of the pharmacological activity of Nortriptyline in CADosome formulations using a dual-luciferase assay. (A) Schematic diagram of the dual-luciferase assay. (B) Percentage of 5HT2AR activation induced by blank, free NT (30μM, 100μM), and various NT-DOPE doped eGFP-mRNA loaded CADosomes, measured by calculating the area under the receptor activation curve (AUC) value. 1μM of LSD was added to all samples, and luminescence was monitored continuously for 2 hours. n=3. (C-E) Representative activation spectra for blank, 30μM free NT, and mRNA NT-doped N/P9.
This article also evaluated the high transfection efficiency of CADosomes using HeLa reporter cell lines stably expressing the Cre reporter plasmid pLV-CMV-LoxP-DsRed-LoxP-eGFP and primary bovine corneal epithelial cells (PBCECs). Finally, the safety and efficacy of CADosomes for local nucleic acid delivery to the cornea were demonstrated through eye drop administration in a rabbit eye model (as shown in Figure 3).
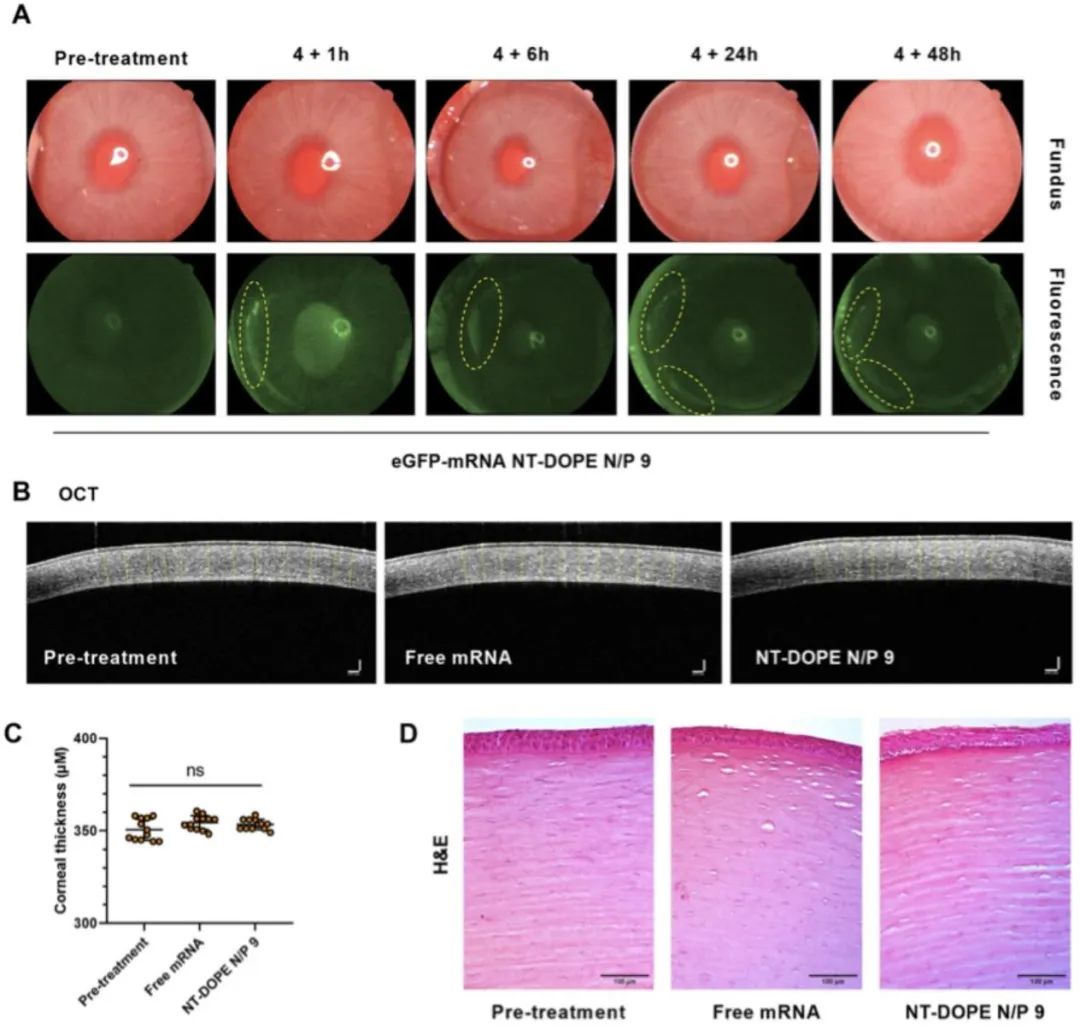 Figure 3. In vivo delivery of eGFP-mRNA in a rabbit eye model and evaluation of mRNA CADosomes for corneal toxicity. (A) Color fundus and fluorescence images of rabbit eyes treated with eGFP-mRNA NT-DOPE N/P9 CADosomes at a final concentration of 2μg/ml, with yellow circles marking areas of high eGFP expression at different time points post-treatment. (B-C) Two-dimensional optical coherence tomography (OCT) images showing corneal thickness before and after administration, with no significant changes observed. The scale bar corresponds to 100μm. (D) Histological images of corneal surface treated with free eGFP-mRNA and eGFP-mRNA CADosomes.
Overall, the research team demonstrated that tricyclic cationic amphiphilic drugs (CADs) can serve as structural and functional components of lipid nanoparticles, successfully replacing cationic or ionizable lipids. The findings not only provide a new mRNA delivery platform but may also facilitate research on new methods for combination drug therapy.
1. Proposed a universal mRNA delivery platform CADosomes;
2. Confirmed that cationic amphiphilic drugs forming CADosomes still possess pharmacological activity;
3. Demonstrated that the different structures of cationic amphiphilic drugs in the formulation affect cellular uptake and transfection efficiency;
4. Proposed a new strategy for combination drug therapy.
Volume 350, October 2022, Pages 256-270
https://doi.org/10.1016/j.jconrel.2022.08.009
Corresponding author, Dr. Koen Raemdonck, is a professor at the University of Ghent, Belgium. Previously, he was a visiting postdoctoral researcher at ETH Zurich and holds a PhD in pharmaceutical sciences. His research focuses on exploring new biomimetic approaches to deliver therapeutic macromolecules across intracellular and extracellular biological barriers. Currently, Dr. Koen Raemdonck is working on inhalable RNA formulations for treating lung diseases.
Translation | Wang Yuwei, Xu Yanhua
Typesetting | Wang Hairui
This article is original to J Control Release, and personal sharing is welcomed. Any other media or website wishing to reprint must indicate the source J Control Release WeChat account before the text.
Figure 3. In vivo delivery of eGFP-mRNA in a rabbit eye model and evaluation of mRNA CADosomes for corneal toxicity. (A) Color fundus and fluorescence images of rabbit eyes treated with eGFP-mRNA NT-DOPE N/P9 CADosomes at a final concentration of 2μg/ml, with yellow circles marking areas of high eGFP expression at different time points post-treatment. (B-C) Two-dimensional optical coherence tomography (OCT) images showing corneal thickness before and after administration, with no significant changes observed. The scale bar corresponds to 100μm. (D) Histological images of corneal surface treated with free eGFP-mRNA and eGFP-mRNA CADosomes.
Overall, the research team demonstrated that tricyclic cationic amphiphilic drugs (CADs) can serve as structural and functional components of lipid nanoparticles, successfully replacing cationic or ionizable lipids. The findings not only provide a new mRNA delivery platform but may also facilitate research on new methods for combination drug therapy.
1. Proposed a universal mRNA delivery platform CADosomes;
2. Confirmed that cationic amphiphilic drugs forming CADosomes still possess pharmacological activity;
3. Demonstrated that the different structures of cationic amphiphilic drugs in the formulation affect cellular uptake and transfection efficiency;
4. Proposed a new strategy for combination drug therapy.
Volume 350, October 2022, Pages 256-270
https://doi.org/10.1016/j.jconrel.2022.08.009
Corresponding author, Dr. Koen Raemdonck, is a professor at the University of Ghent, Belgium. Previously, he was a visiting postdoctoral researcher at ETH Zurich and holds a PhD in pharmaceutical sciences. His research focuses on exploring new biomimetic approaches to deliver therapeutic macromolecules across intracellular and extracellular biological barriers. Currently, Dr. Koen Raemdonck is working on inhalable RNA formulations for treating lung diseases.
Translation | Wang Yuwei, Xu Yanhua
Typesetting | Wang Hairui
This article is original to J Control Release, and personal sharing is welcomed. Any other media or website wishing to reprint must indicate the source J Control Release WeChat account before the text.