Sonodynamic Therapy (SDT) is a novel non-invasive tumor treatment method that utilizes ultrasound to activate sonosensitizers to generate reactive oxygen species (ROS) to induce tumor cell death. Based on the deep tissue penetration capability of ultrasound, SDT can treat deep tumors and has broad clinical transformation prospects. The main mechanism of SDT is the generation of ROS from oxygen by ultrasound, leading to apoptosis or necrosis of cancer cells. However, the hypoxic microenvironment of tumors limits ROS production and activates protective autophagy processes that protect tumor cells from oxidative stress. Therefore, increasing ROS production and inhibiting protective autophagy during sonodynamic therapy will help improve the sensitivity of SDT treatment.

Recently, Researcher Zhang Mingzhen’s team from Xi’an Jiaotong University and Director Zhang Mingxin of the Department of Gastroenterology at the First Affiliated Hospital of Xi’an Medical University along with Researcher Fan Kelong from the Chinese Academy of Sciences collaborated to synthesize a cascaded nano-reactor that enhances sonodynamic therapy for colon cancer by synergistically increasing ROS and blocking autophagy. First, the autophagy inhibitor chloroquine and the sonosensitizer Ce6 were loaded into a hollow polydopamine nano-carrier (CCP@HP) pre-doped with platinum nanoenzymes. Next, homologous cell membranes were used to functionalize the surface of the nano-carrier (CCP@HP@M) to help the nano-drug delivery system better target the tumor site, achieving more significant therapeutic effects. The polydopamine nano-carrier (HP) has superoxide dismutase activity, which can convert O2.– into O2 and H2O2, while platinum nanoenzymes further catalyze H2O2 to produce toxic ·OH and O2. Under ultrasound irradiation, CCP@HP@M can effectively alleviate the hypoxic state of tumors, enhance ROS generation, inhibit protective autophagy pathways, and induce apoptosis and ferroptosis (Figure 1). This cascaded nano-reactor provides a new strategy for precise treatment of deep tumors by modulating ROS and autophagy to enhance SDT.
First, the hollow polydopamine doped with Pt nanoenzymes was characterized by TEM, elemental analysis, particle size distribution, Zeta potential, and XRD. Next, the successful coating of the cell membrane was verified by Coomassie blue and WB. The SOD enzyme activity of HP and the POD and CAT enzyme activities of Pt nanoenzymes were also confirmed (Figure 2).
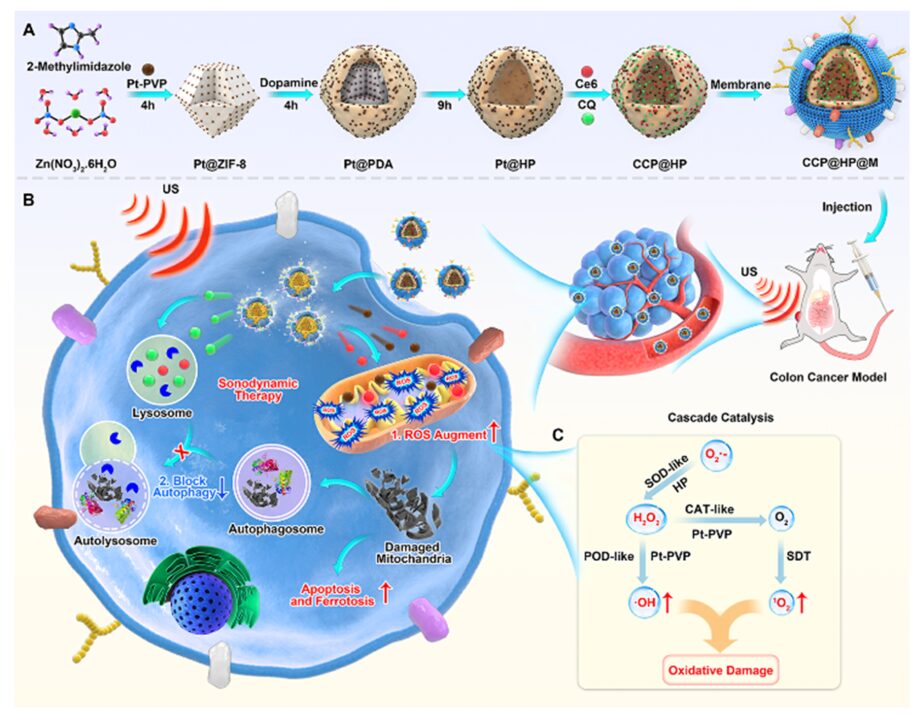
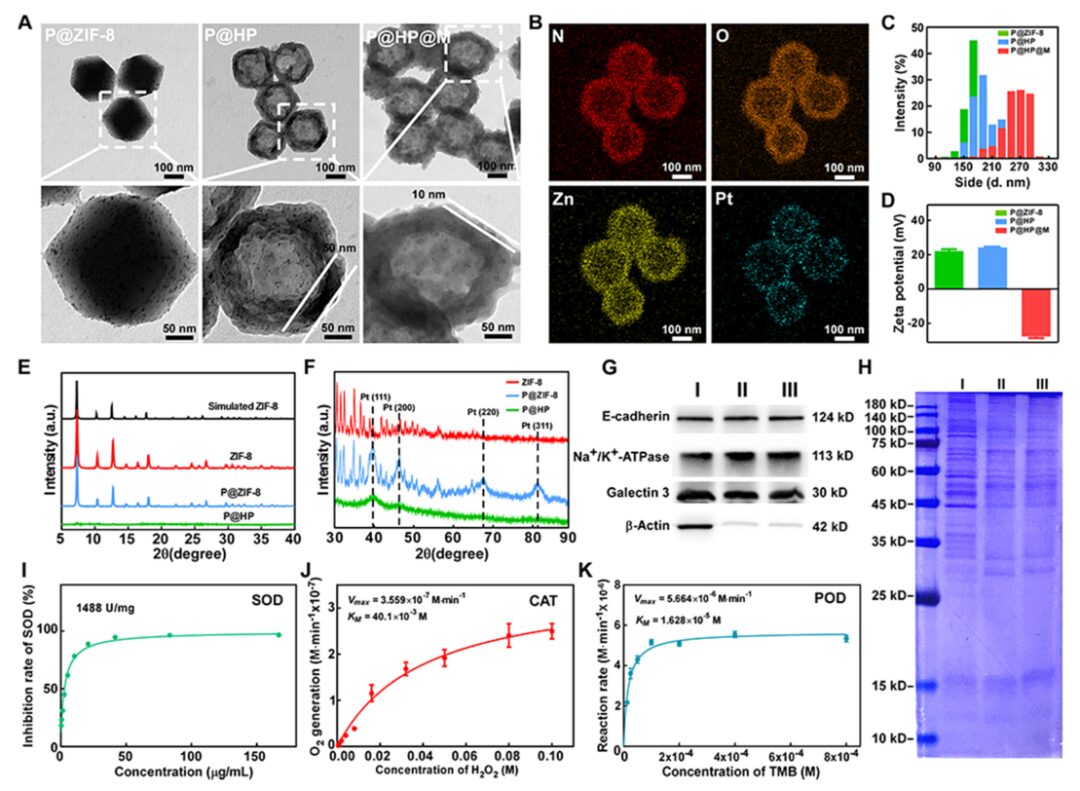
Figure 2: Characterization of P@HP@M.
Next, the drug loading situation of CCP@HP@M was characterized. UV absorption spectra indicated the successful co-loading of Ce6 and CQ, and the encapsulation rate and release were also tested. Considering the inhibitory effect of hypoxia at the tumor site on SDT efficiency, the CAT enzyme-like activity of CCP@HP was tested, along with the dissolved O2 levels. The generation of 1O2 and ·OH was also detected, proving that CCP@HP can effectively enhance the level of ROS generation in ultrasound treatment (Figure 3).
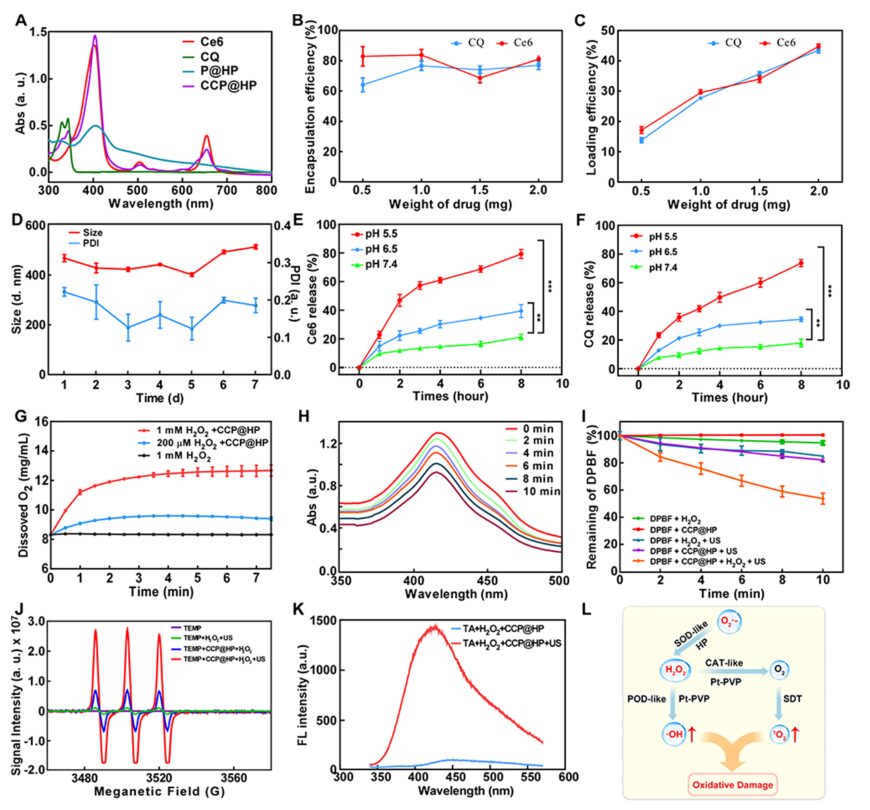
Figure 3: Characterization of CCP@HP@M.
Ce6 has good near-infrared fluorescence, which can be used for fluorescence imaging. By tracking the fluorescence signal of Ce6 through microscopic fluorescence imaging, the cellular uptake and targeting capability of CCP@HP@M were studied. The subcellular localization of Ce6 was tracked to determine the expression of CCP@HP@M in HCT-116 cells, revealing that CCP@HP@M enters HCT-116 cells through lipid membrane fusion and receptor-mediated endocytosis. The substantial accumulation of CCP@HP@M in the mitochondria of HCT-116 cells enhances the efficiency of SDT; additionally, accumulation in lysosomes also aids in blocking autophagy (Figure 4).
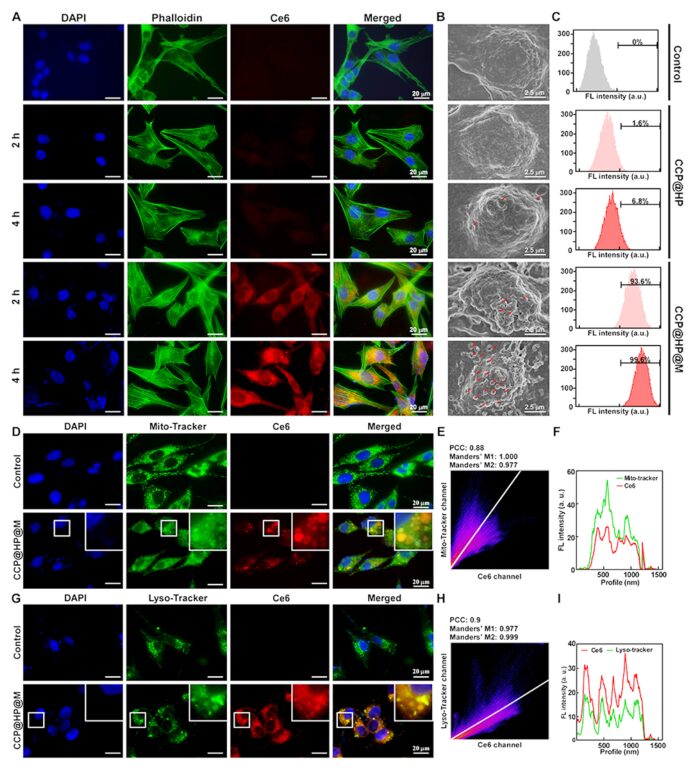
Figure 4: Cellular uptake and intracellular distribution of CCP@HP@M.
To investigate the therapeutic effects of the nano-reactor, the efficacy of different treatment methods was assessed using AM/PI and MTT assays, and cell proliferation was analyzed through colony formation assays, while cell migration was analyzed via scratch assays. The results indicated that combination treatment significantly inhibited the proliferation and migration of HCT-116 cells (Figure 5).
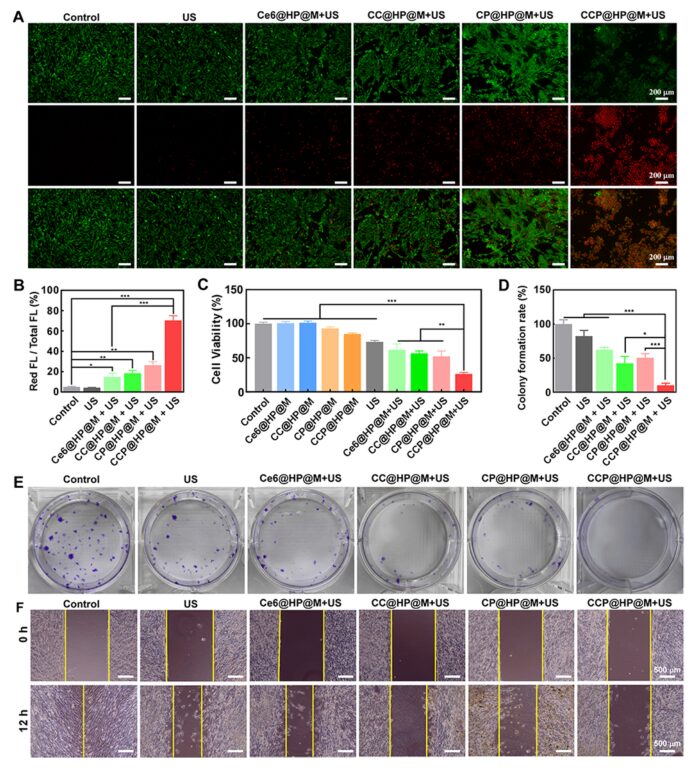
Figure 5: In vitro therapeutic effects.
Next, the ability of the nano-reactor to enhance ROS generation and alleviate intracellular hypoxia was assessed. When ROS attack the mitochondrial membrane, the mitochondrial membrane potential (MMP) is lost, leading to the release of apoptosis-inducing factors and inducing mitochondrial-mediated tumor cell apoptosis. The mitochondrial membrane potential (MMP) was assessed using JC-1, and the results indicated that ROS levels increased in HCT-116 cells, while MMP decreased. The loss of MMP is an early marker of apoptosis, and flow cytometry analysis also indicated that the cascaded catalytic nano-reactor induced the apoptotic pathway in HCT-116 cells. Class POD nanoenzymes can effectively convert less reactive H2O2 into ·OH, thereby significantly enhancing the ferroptosis treatment effect of cancer cells. The typical feature of ferroptosis is the accumulation of lipid peroxides, which were detected in the CCP@HP@M+US group. These results indicate that the nano-reactor CCP@HP can induce ferroptosis. To further confirm the mechanism of the cascaded nano-reactor combined with SDT on colon cancer cells, ROS inhibitors and specific apoptosis and ferroptosis inhibitors were used to evaluate their blocking effects. Finally, to study the mechanism by which the autophagy inhibitor CQ improves SDT, Western blot analysis was performed to assess autophagy flux, and the results found that inhibiting protective autophagy significantly improved the efficiency of SDT by activating ferroptosis and apoptosis (Figure 6).
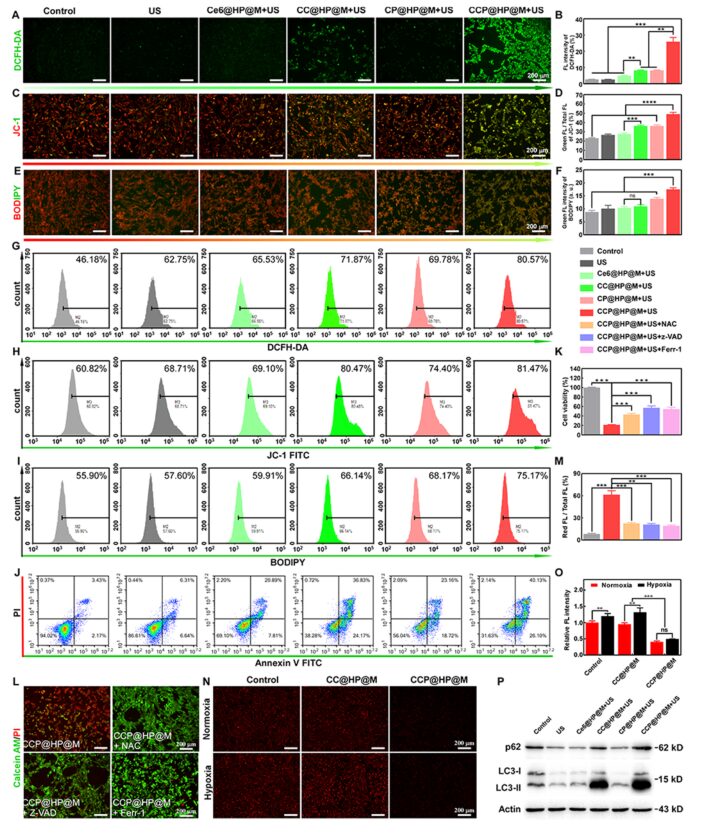
Figure 6: The CCP@HP@M nano-reactor effectively increases ROS production and induces apoptosis and ferroptosis.
The in vitro cytotoxicity of the nano-reactor was assessed through MTT and hemolysis assays, and in vivo biocompatibility was evaluated through blood biochemistry, blood routine tests, and HE staining, confirming that CCP@HP@M has outstanding biosafety (Figure 7).
By capturing fluorescence images to monitor the accumulation of the nano-reactor in tumors, it was found that the signal peaked 3 hours after injection, indicating effective enrichment of the nano-reactor at the tumor site, effectively guiding the timing of US irradiation. Pharmacokinetic results showed that the nano-reactor could be metabolized from the mice, and CCP@HP@M not only effectively accumulates at the tumor site, ensuring combined treatment of the tumor but can also gradually be metabolized out of the body, demonstrating good biosafety (Figure 8).
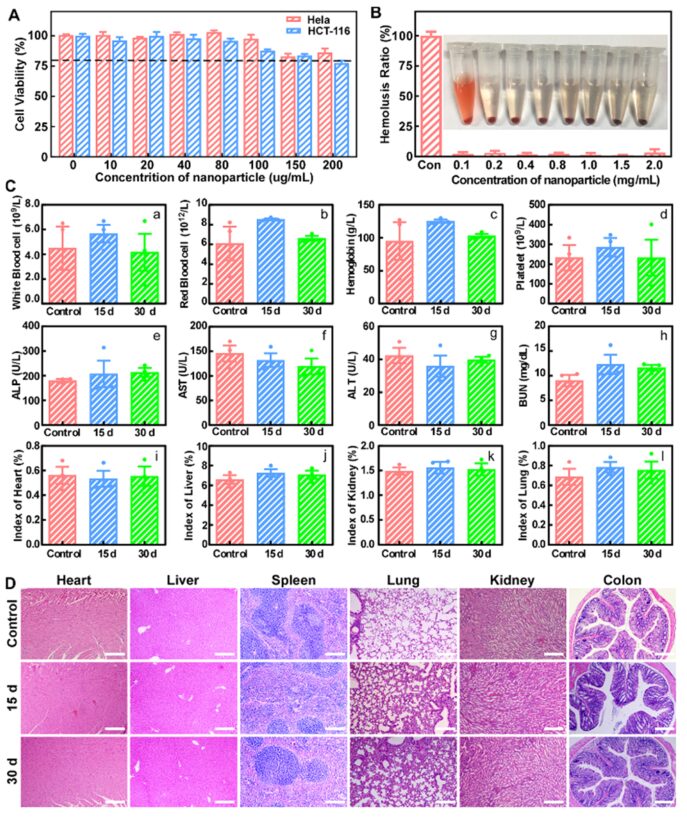
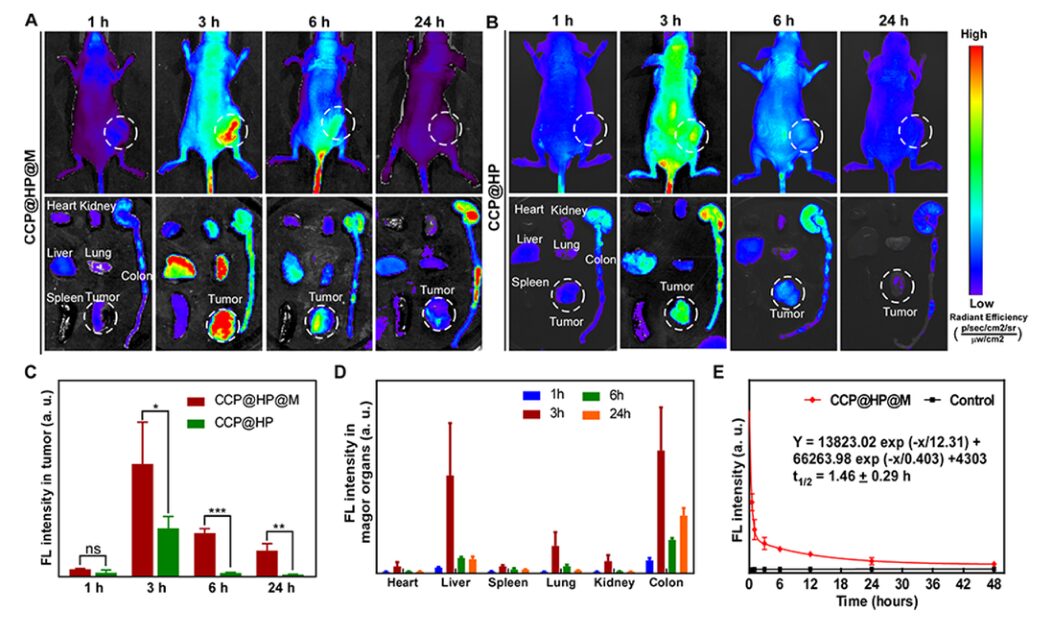
Figure 8: Biodistribution and pharmacokinetics of CCP@HP@M.
Animal experiment results showed that the combination treatment group (CCP@HP@M+US) exhibited excellent anti-tumor effects. Using vascular markers anti-CD31 and hypoxia markers anti-HIF-1α to stain tumor cells, it was confirmed that Pt nanoenzymes can continuously decompose H2O2 into O2 at the tumor site to alleviate hypoxia. To study the autophagy blockade of the nano-reactor, tumor cells were stained with autophagosome markers LC3 and autophagy substrate p62, indicating that autophagy was induced in the combination treatment group (CCP@HP@M+US). TUNEL staining analysis showed the level of apoptosis in tumor cells under different treatments, indicating that the cascaded catalytic nano-reactor can effectively improve the therapeutic effect of SDT on tumors (Figure 9).
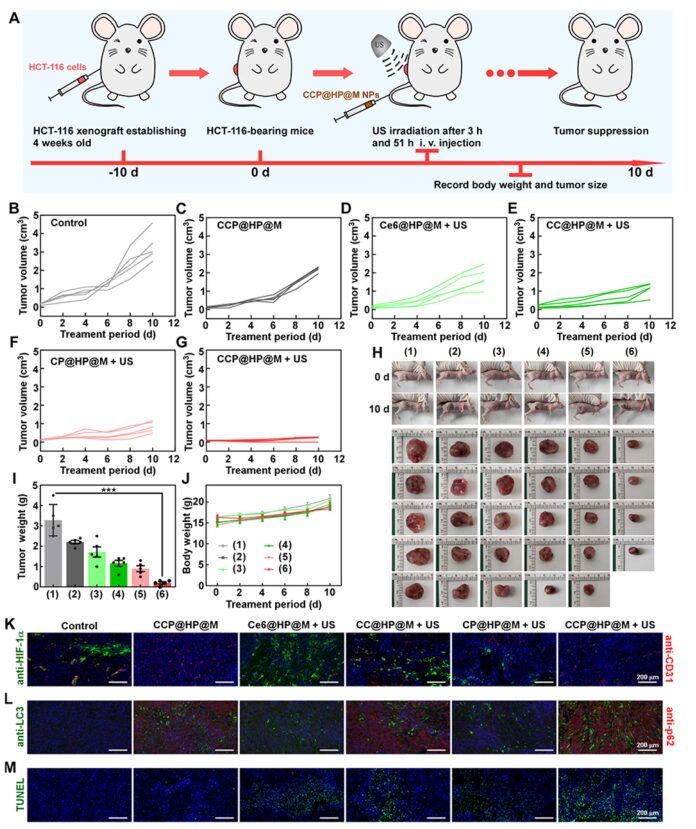
Figure 9: In vivo combined therapeutic effects of CCP@HP@M.
To further explore the potential mechanisms of the nano-reactor in the treatment of colon cancer in vivo, RNA-seq analysis was conducted. RNA-seq results showed 232 differentially expressed genes, of which 74 were upregulated, and 158 were downregulated. Among the upregulated genes, most were related to transmembrane transport proteins in the GO database, indicating that the interaction between the nano-reactor and cells is mediated by membrane transport proteins. KEGG analysis indicated that various autophagy pathways, apoptosis pathways, ferroptosis pathways, and HIF-1α were downregulated, which aligns with the enhanced treatment effect on colon cancer through alleviating hypoxia and modulating autophagy to induce apoptosis and ferroptosis by the nano-reactor (Figure 10).
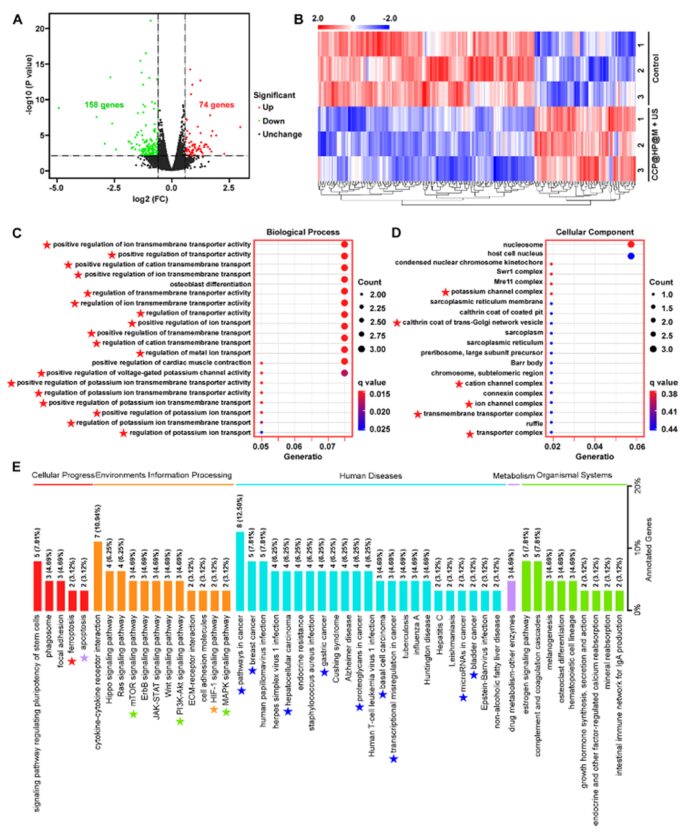
Figure 10: RNA-seq analysis revealing the tumor treatment mechanism.
[Conclusion]
This study developed a biomimetic cascaded catalytic nano-reactor CCP@HP@M, a hollow polydopamine nano-reactor doped with Pt nanoenzymes, encapsulating CQ and Ce6 through cell membrane wrapping. By optimizing sonodynamic therapy for colorectal cancer through ROS and autophagy modulation strategies, the nano-reactor CCP@HP@M demonstrates good pH responsiveness, targeting ability, biodegradability, and biocompatibility. CCP@HP@M can enhance SDT efficiency by increasing ROS production and blocking autophagy flux, thereby inducing apoptosis and ferroptosis. This study presents a promising therapeutic method to improve SDT efficiency. Additionally, this work reveals the good synergistic effect of SDT and autophagy inhibitors in the treatment of colorectal cancer.
https://www.sciencedirect.com/science/article/abs/pii/S1748013223000476