
iNature
Glioblastoma (GBM) has an immune-suppressive microenvironment and blood-brain barrier (BBB) that severely hinders the infiltration and activity of natural killer (NK) cells, thereby reducing their clinical efficacy in GBM treatment.
On December 23, 2024, Zhang Xianzheng from Wuhan University published a study titled “Cationic Magnetic Nanoparticles Activate Natural Killer Cells for the Treatment of Glioblastoma” in ACS Nano.To address these challenges, this study introduced an engineered living material HEFDS-NK cells, aimed at enhancing NK cell permeability across the BBB and improving their cytotoxicity against GBM.
HEFDS consists of magnetic nanoparticles modified with cationic polyethyleneimine (PEI), selenocysteine (Sec), and sodium hyaluronate (HA), co-cultured with NK cells to form HEFDS-NK cells. With the help of HA and magnetic targeting, HEFDS-NK cells can effectively cross the BBB and localize at GBM sites. Additionally, PEI enhances the expression of C-X-C chemokine receptor type 4 (CXCR4) and C-C chemokine receptor type 4 (CCR4) on NK cells, improving their recognition and cytotoxicity against GBM. Furthermore, Sec enhances the immune activity of NK cells. Upon recognizing GBM, HEFDS-NK cells activate and produce granzyme B, perforin, and IFN-γ, effectively treating GBM. This study provides an innovative therapy that enhances NK cell activity and BBB penetration while effectively treating GBM.

In recent years, immunotherapy using T lymphocytes and NK cells has emerged as an effective cancer treatment. Among them, NK cells have good safety, avoiding complications such as cytokine release syndrome and graft-versus-host disease, which gives them an advantage over T lymphocytes. As a key component of the innate immune system, NK cells play a critical role in the initial defense against cancer cells by releasing granzyme B, perforin, and IFN-γ upon activation. Clinical evidence shows that NK cell activation is negatively correlated with cancer incidence, making NK cells a potential ‘living drug’ in cancer treatment. However, challenges such as insufficient NK cell infiltration and activity due to the BBB and the immune-suppressive microenvironment of GBM lead to poor clinical outcomes in GBM treatment.
The BBB consists of brain endothelial cells, pericytes, astrocytes, and the basement membrane, forming a highly selective semipermeable membrane that regulates material exchange between blood and the central nervous system (CNS).While the BBB protects the CNS from harmful elements, it also hinders the delivery of therapeutic drugs and the infiltration of immune cells. For instance, the BBB obstructs NK cells from effectively infiltrating GBM sites, affecting treatment efficacy. Current strategies for penetrating the BBB, such as focused ultrasound, microwaves, and lasers, carry risks of damaging barrier integrity and increasing CNS susceptibility to harmful substances. Therefore, there is an urgent need for alternative methods to enhance NK cell infiltration without compromising BBB integrity. Additionally, the immune-suppressive microenvironment of GBM inhibits NK cell activity, further reducing their therapeutic effect. The study emphasizes the importance of NK cell surface markers such as CXCR4 and CCR4 in recognizing and eliminating GBM. Specifically, CXCR4 overexpression enhances NK cell immunotherapy against GBM, while CCR4 enhances NK cell responses to activated regulatory chemokines. These findings highlight the potential of targeting NK cell activation strategies in GBM treatment.
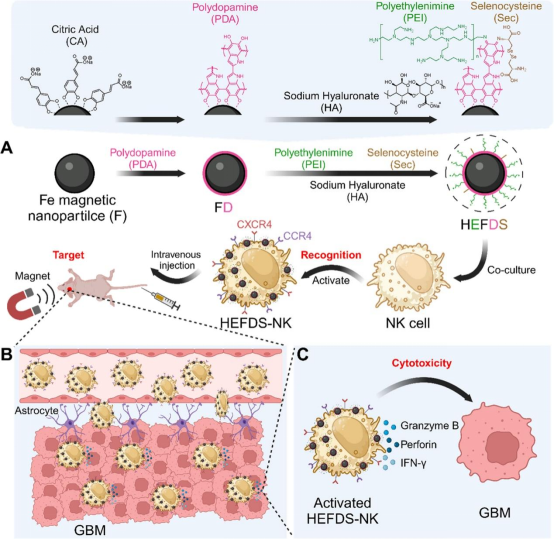
Figure 1: Schematic diagram of cationic magnetic nanoparticles activating natural killer cells for glioblastoma treatment (adapted from ACS Nano)
The combination of natural biological components and synthetic elements is rapidly developing in the field of engineered living materials (ELM). ELM enhances NK cell function by imparting specific functionalities and reactivities to NK cells, showing great potential in GBM treatment. This study developed an ELM named HEFDS-NK cells to address the issues of NK cell infiltration and activity insufficiency in GBM. HEFDS nanoparticles were synthesized by modifying the surfaces of magnetic nanoparticles with polyethyleneimine (PEI), selenocysteine (Sec), and sodium hyaluronate (HA). These modifications facilitated the electrostatic interaction that promoted the adsorption of positively charged HEFDS nanoparticles onto negatively charged NK cells, forming HEFDS-NK cells. After intravenous injection, HEFDS-NK cells cross the BBB and accumulate at GBM sites with the help of HA and magnetic guidance. PEI modification enhances the expression of CXCR4 and CCR4 on NK cells, improving their recognition and cytotoxicity against GBM. SEC modification further enhances the immune activity of NK cells. Upon recognizing GBM, activated HEFDS-NK cells produce high levels of granzyme B, perforin, and IFN-γ at the tumor site, achieving effective GBM treatment. This study introduces an innovative approach utilizing ELMs to overcome the challenges of NK cell infiltration and activity in current GBM treatments.
References:
https://pubs.acs.org/doi/10.1021/acsnano.4c11250

—END—
The content is original from 【iNature】,
Reprint must indicate the source from 【iNature】
Add WeChat Group
iNature gathers 40,000 life science researchers and doctors. We have formed 80 comprehensive groups (16 PI groups and 64 PhD groups), and also established specialized groups in related fields (plants, immunology, cells, microbiology, gene editing, neuroscience, chemistry, physics, cardiovascular, oncology, etc.).Kind reminder: Please note when joining the group (format: school + major + name; if you are a PI/professor, pleaseindicate as PI/professor, otherwise it will be assumed you are a PhD student. Thank you).You can first add the editor’s WeChat ID (love_iNature), or long press the QR code to add the editor, and then join the relevant group. Serious inquiries only.

Submission, collaboration, and reprint authorization matters
Please contact WeChat ID:13701829856 or email:[email protected]
If you find this article interesting, please click here!