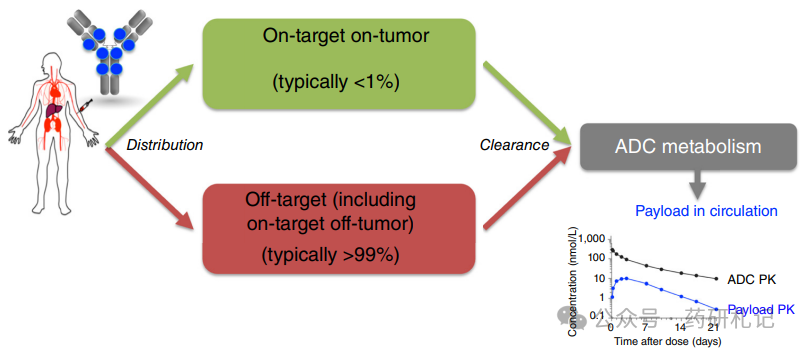
The Metabolism Process of ADC
The in vivo delivery process of the payload is complicated due to the complex structure of ADCs and their metabolic degradation in the body. After ADC administration, the Drug-to-Antibody Ratio (DAR), bound or free antibodies, albumin conjugates, free payloads, and payload metabolites are all in a highly dynamic state over time. Unlike small molecule drugs that are primarily excreted directly, the disposition of ADCs mainly relies on metabolic processes. The released payload and/or its metabolites are eliminated through classical small molecule pathways. Similar to all antibody drugs, ADCs are primarily distributed in normal tissues, leading to significant off-tumor uptake and clearance. In other words, the main metabolic pathway of ADCs is non-target dependent uptake in normal tissues. For ADCs containing multiple DAR components, the rate and location of non-specific uptake may be DAR-dependent, with higher DAR components typically cleared more rapidly. For some targets that are widely expressed in normal tissues, the disposition of ADCs mainly manifests as “on-target off-tumor” uptake, in addition to non-specific uptake. Targeted (both intra-tumor and extra-tumor) and non-targeted ADCs metabolize and degrade in tissues (including small amounts of tumor tissue) to produce free payloads and/or their metabolites, which are then redistributed into circulation (as shown in the figure below).
Moreover, the instability of some linkers can directly lead to the release of free payloads or the transfer of drug-linkers from ADCs to albumin, forming albumin conjugates. In humans, the plasma concentration of payloads derived from ADC metabolism and linker instability typically exceeds the in vitro effective free drug concentration (EC50) over time. If the payload is administered as a small molecule chemotherapy drug, exposure above EC50 will last only a few minutes to a few hours rather than days, highlighting the role of ADCs as payload reservoirs. In a sense, ADCs act as prodrugs for their payloads, with the payload being released over a longer duration relative to the ADC half-life, resembling a “continuous infusion” pattern.
Although it remains uncertain to what extent free payloads contribute to the anti-tumor effects in individual cases, it is reasonable to speculate that, in addition to ADC-targeted delivery, free payloads do have additional contributions. These mechanisms indicate the unique role of antibodies in enhancing the efficacy of conjugated drugs, which relies not only on tumor-specific ADC uptake but also on other contributions. In fact, the interaction between tumor uptake and off-tumor targeting/non-targeting uptake, combined with linker instability, maintains drug concentrations in the body and at tumor sites. It should be clarified that while only a small portion of the ADC dose reaches the tumor, the off-tumor released payloads do not necessarily accumulate in the tumor, with the vast majority distributed and eliminated through classical small molecule pathways in normal tissues, similar to traditional chemotherapy. However, the plasma concentration of released payloads can maintain effective levels for extended periods, which may contribute significantly to efficacy and explain certain payload-related systemic toxicities.
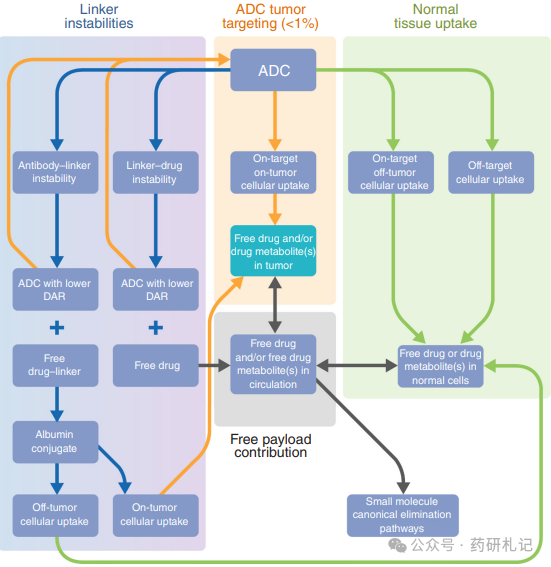
Additionally, linker instability leads to a gradual decrease in the drug load of antibody components in circulation over time; as long as they carry at least one drug load, they still belong to the ADC. ADCs with lower DAR typically exhibit better pharmacokinetic (PK) characteristics and different normal tissue uptake profiles, but each antibody carries less payload. For ADCs with completely stable linkers, there are no low drug load antibody components in circulation, and ADCs rely primarily on normal tissue (mainly) or tumor site (usually <1%) uptake to release payloads. These phenomena await clinical validation to clarify the balance of ADC efficacy and toxicity, likely depending on multiple factors and specific case analyses. As mentioned, ADCs with stable linkers often exhibit unexpected toxicities, possibly due to the inevitable uptake of ADCs into normal tissues.
Systemic Distribution of ADCs: Insights from Radioactive Labeling Molecular Imaging
Positron Emission Tomography (PET) combined with radioactive tracers can provide deep insights into the in vivo distribution of ADCs (and other antibody drugs). Antibody imaging studies commonly use the radioactive nuclide Zirconium-89 (89Zr), whose 78.4-hour half-life matches the slow clearance characteristics of antibodies. Clinical PET distribution studies of 89Zr-labeled antibodies (such as 89Zr-trastuzumab, 89Zr-MMOT0530A, 89Zr-bevacizumab, and 89Zr-trastuzumab) have shown that unless the tumor burden is extremely high, only a small amount of antibodies is taken up by the tumor. The distribution patterns of off-tumor targeting and non-targeting of the tracers on day 4 post-injection are highly similar: about 1/3 of the tracer exists in the circulatory system, up to 15% in the liver, 4% in the spleen and kidneys; concentrations in bone marrow, lungs, dense bones, muscles, adipose tissue, and brain are lower. However, the low accumulation of tracers per gram of tissue may still be influenced by large volume tissues, especially adipose tissue, with an average of about 5% to 7% accumulating in adipose tissue, but this proportion can increase to 19% in cases of morbid obesity.
Notably, the amount of antibody accumulated in tumor lesions per patient is usually less than 1%. Studies with 89Zr-trastuzumab indicate that in a patient population with a median measurable tumor burden of 99 (±133) mL, the average tumor uptake rate is 0.9% ID. The standard human volume is about 70 liters, and a 0.9% ID distribution in 99 mL of tumor tissue indicates significant tumor-selective uptake, as the tumor volume only accounts for 0.1%. This finding aligns with multiple clinical studies of radioactive labeled antibodies: although the standardized uptake value (SUV) of radioactive conjugates within tumors is high, the absolute uptake amount is usually low (generally <1% ID), primarily distributed in normal tissues.
Tumor volume and location also affect antibody distribution. For example, in a patient with bone metastasis accompanied by a 1.2 kg liver metastatic tumor, the large tumor burden significantly impacts the PK of trastuzumab. PET imaging 2 days after injecting 50 mg of 89Zr-trastuzumab (containing 1.5 mg of radioactive antibody + 48.5 mg of non-radioactive antibody) showed that 48% of the dose concentrated in the liver metastatic site, with rapid clearance from circulation. Therefore, the dosage of trastuzumab and HER2-targeted ADCs in metastatic breast cancer patients may be significantly influenced by tumor burden—this also explains the inter-patient PK differences, as shown with T-DM1.
The phenomenon of Target-Mediated Drug Disposition (TMDD) is common with antibody drugs, leading to increased clearance at low doses and nonlinear PK. At high doses, the antigen pool becomes saturated, and the antibody exposure increases proportionally. For example, margetuximab shows nonlinear increases in AUC at doses of 0.1, 0.3, 1.0, and 3.0 mg/kg weekly, while at doses of 10, 15, and 18 mg/kg every three weeks, it shows linear increases.
These insights regarding the distribution and disposition of ADCs in normal tissues profoundly influence strategies to improve tumor penetration and reduce toxicity. In an early clinical trial of the EGFR antibody-dye conjugate panitumumab-IRDye800CW, the combined use of unconjugated panitumumab improved the intra-tumor distribution of panitumumab-IRDye800CW. Microscopic examination after combination therapy showed improved micro-distribution in tumors and reduced uptake in healthy tissues. The authors thus proposed that the payload dose may lower the binding site barrier, enhancing the tissue penetration of antibody-dye conjugates (which may also apply to ADCs). Notably, considering the sustained uptake observed during PET scans of trastuzumab and HER3 antibody margetuximab treatment (respectively 89Zr-trastuzumab and margetuximab), achieving complete saturation of antibodies within tumors is challenging. This may be due to the continuous production and recycling of antibody targets by tumor cells.
In the field of hematologic malignancies, the understanding of systemic distribution of antibodies has influenced pre-targeting strategies for radioimmunotherapy (RIT) and bispecific antibodies. To improve the therapeutic index of RIT, researchers have proposed administering excess unlabeled antibodies prior to RIT. This preloading strategy aims to prevent non-specific binding in normal tissues or saturation of normal cells expressing the target, ensuring a more consistent distribution of radioactive labeled antibodies and prolonging half-life by reducing clearance rates. Taking rituximab as an example, preloading with unlabeled antibodies can reduce the number of circulating B cells, enhancing the delivery of radioactive labeled antibodies to tumor cells. However, another biodistribution study showed that tumor uptake was higher in patients without preloading, indicating that this strategy requires further optimization.
Compared to tumor biopsies, antibody PET imaging can provide target expression information for all lesions in patients. Numerous studies have shown significant heterogeneity in antibody tracer uptake within and between patients. To explore how this heterogeneity affects ADC efficacy, researchers evaluated the value of HER2-targeted molecular imaging in screening T-DM1 non-responders. HER2-positive breast cancer patients underwent baseline 89Zr-trastuzumab PET/CT and 18F-FDG PET-CT scans before T-DM1 treatment. Lesions were categorized based on PET uptake as HER2-positive (visible/high uptake) and negative (background/nearly background activity). Among 81 patients, 26 (despite biopsy-confirmed HER2 positivity) were PET-negative, and these patients had significantly shorter time to treatment failure (TTF, median 2.8 vs 9.9 months) compared to PET-positive patients (HR 3.7, P<0.001). If T-DM1 treatment were selected based solely on PET HER2 positivity, 320 out of 1000 patients would have gone untreated.
Recent studies indicate that patients with low or no HER2 expression may also respond to HER2-targeted ADCs. In a prospective multicenter IMPACT trial, 189 newly diagnosed metastatic breast cancer patients were assessed for HER2 status through tumor biopsies and baseline 89Zr-trastuzumab PET. Significant intra- and inter-patient lesion uptake heterogeneity was observed across all HER2 IHC groups. PET detected HER2-positive metastatic lesions in HER2-negative disease and negative metastatic lesions in HER2-positive disease, with even uptake exceeding background in HER2 IHC low-expressing/negative metastatic lesions. These findings suggest that 89Zr-trastuzumab PET may optimize patient selection for novel HER2-targeted strategies. Shorter half-life radioactive nuclides (such as 68Ga-labeled single-domain antibodies, affibodies, and cyclic peptides) also show promise in HER2 and nectin-4 imaging. Finally, site-specific radioactive labeled antibodies have demonstrated superior performance in preclinical studies compared to randomly modified parent antibodies. If this advantage can be replicated in clinical settings, it may ultimately benefit both patients and physicians by guiding targeted therapies like ADCs.
References:Raffaele Colombo, Paolo Tarantino, Jamie R. Rich, Patricia M. LoRusso, Elisabeth G.E. de Vries; The Journey of Antibody–Drug Conjugates: Lessons Learned from 40 Years of Development. Cancer Discov 1 November 2024; 14 (11): 2089–2108