Professor Yu Zhiqiang from Southern Medical University and Researcher Tang Longguang from Zhejiang University published a research paper titled “pH-activated nanoplatform for visualized photodynamic and ferroptosis synergistic therapy of tumors” in the international academic journal Journal of Controlled Release (IF=11.467).
Compared to traditional chemotherapy, photodynamic therapy (PDT) can effectively reduce side effects and prevent drug resistance. However, there are few reports on amphiphilic photosensitizers with excitation wavelengths in the near-infrared I region (NIR-I) (700-900 nm) that also possess pH-responsive near-infrared II (NIR-II) fluorescence imaging capabilities (1000-1700 nm). NIR-II has become a research hotspot due to its high signal-to-noise ratio, significant tissue penetration, and excellent resolution compared to NIR-I imaging effects. Currently, most photosensitizers have excitation wavelengths in the visible light range (400-700 nm), such as chlorin e6 (Ce6), methylene blue (MB), and hematoporphyrin derivatives (HpD), which exhibit poor tissue penetration and low fluorescence imaging resolution. Additionally, the high content of glutathione (GSH) in tumor tissues significantly reduces the effectiveness of PDT, and a single treatment modality often struggles to cope with the complex tumor microenvironment. The presence of ferroptosis can disrupt the redox homeostasis within tumor cells, reduce intracellular GSH levels, and enhance PDT while further generating •OH to kill cancer cells. Therefore, there is an urgent need for a model that can provide tumor in situ “on/off” responsive NIR-II imaging for PDT and synergistically treat tumors with ferroptosis.
This study reports a novel amphiphilic photosensitizer SR780, which can generate PDT and pH-responsive NIR-II fluorescence and photoacoustic imaging functions under 808 nm excitation. Due to its unique structure, the photosensitizer properties disappear after chelation with Fe3+ (SR780@Fe) and are activated under acidic conditions. Utilizing this interesting phenomenon, the authors designed a pH-activated active targeting nanoparticle based on the synergistic effect of PDT and ferroptosis for multimodal diagnosis and treatment of patient-derived xenograft liver cancer models (PDXHCC).
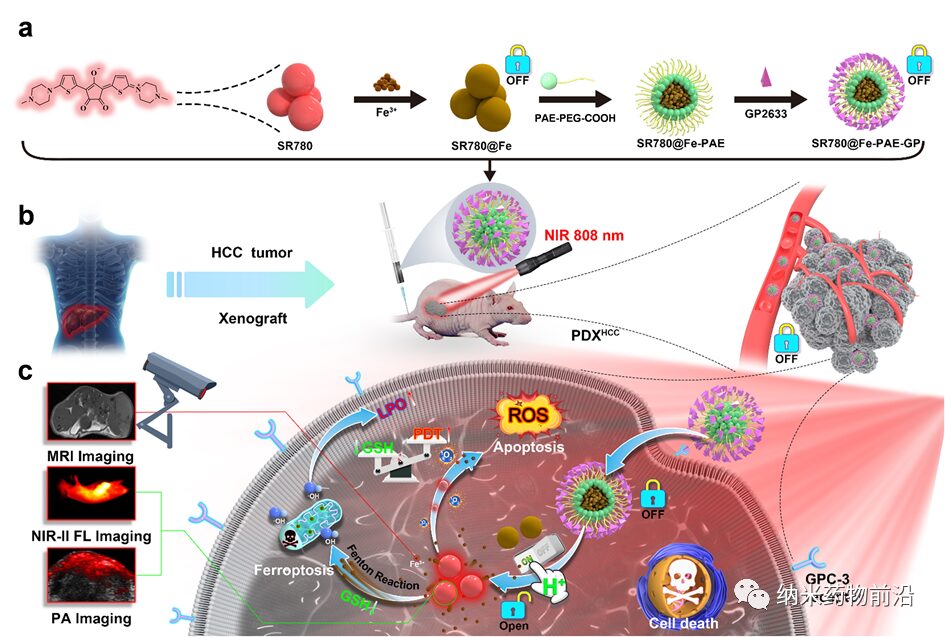
Figure 1. Schematic of the preparation of pH-responsive active targeting nanoparticles with in situ “on/off” functionality and the combination of photodynamic therapy and ferroptosis for liver cancer diagnosis and treatment.
(a) After coordination of SR780 with Fe3+, pH-responsive SR780@Fe-PAE nanoparticles are prepared, which are externally connected with the peptide GP2633 that targets the surface receptor glypican-3 (GPC-3) of liver cancer cells, resulting in a pH-responsive active targeting nanoparticle SR780@Fe-PAE-GP. (b) Establishment of the PDXHCC model and local irradiation of the tumor with 808 nm laser after intravenous administration. (c) After endocytosis of the nanoparticles mediated by the cell surface GPC-3 receptor, the pH-responsive polymer ruptures, activating SR780@Fe in an acidic environment. The released SR780 generates 1O2 under 808 nm laser irradiation, and the acidic conditions enhance NIR-II fluorescence and photoacoustic imaging functions. Furthermore, the released Fe3+ can be used for magnetic resonance imaging (MRI) and enhances PDT effects by consuming GSH. Additionally, •OH generated by the Fenton reaction further kills tumor cells, achieving a good synergistic therapeutic effect of PDT and ferroptosis.
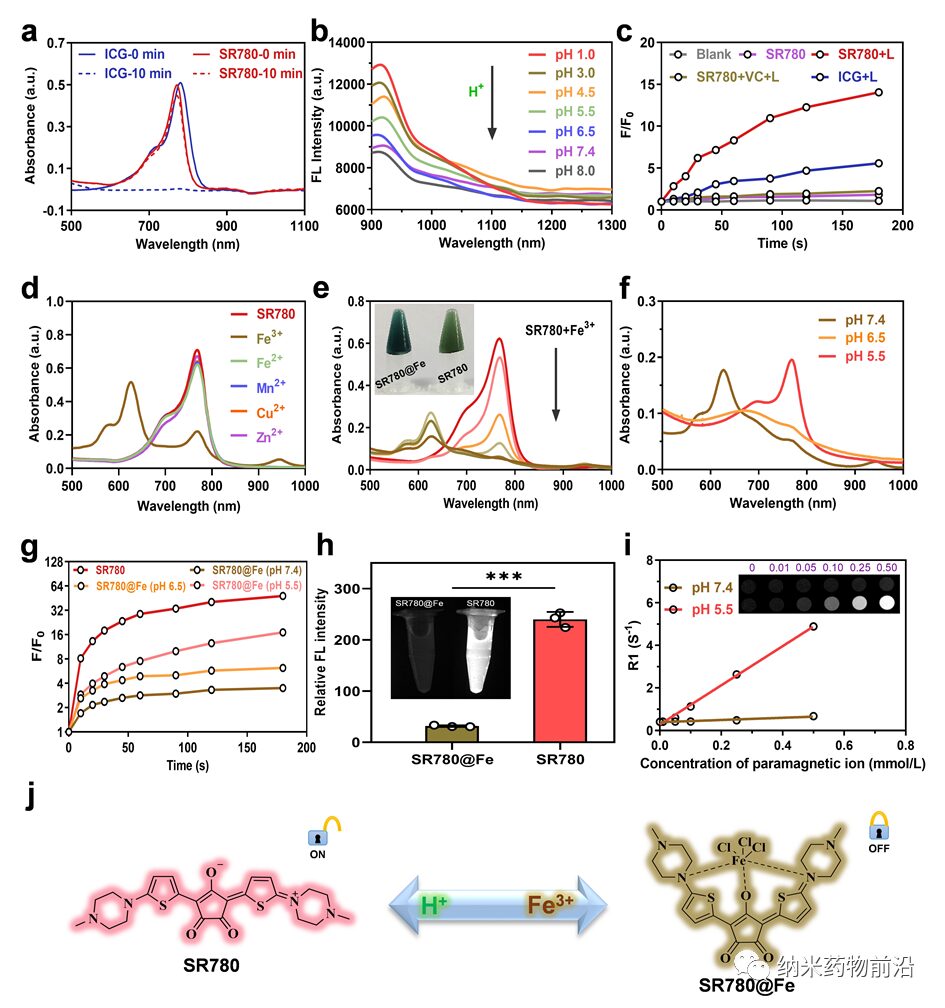
Figure 2. Characterization of the properties of SR780 and SR780@Fe. (a) Comparison of stability between SR780 and ICG at different time points (λ = 808 nm, 1 W cm-2). (b) Changes in NIR-II fluorescence spectra of SR780 under different pH conditions. (c) Comparison of ROS effects generated by SR780 and ICG under the same conditions (λ = 808 nm, 0.5 W cm−2). (d) Investigation of chelation properties between SR780 (20 μM) and different metal ions (Fe3+, Fe2+, Mn2+, Cu2+, Zn2+). (e) Changes in UV absorption after adding (0 ~ 120 μM) Fe3+ to a 10 μM SR780 solution. Insets: Photos of SR780 and SR780@Fe. (f) Changes in UV absorption of SR780@Fe under different pH conditions. (g) ROS generation ability of SR780@Fe at different pH values (λ = 808 nm, 0.5 W cm−2). (h) Relative NIR-II fluorescence imaging signals of SR780 and SR780@Fe. Insets: NIR-II fluorescence imaging photos of SR780 and SR780@Fe. (i) T1 weighted MRI photos and relaxation rate R1 of SR780@Fe at different pH conditions and different Fe3+ concentrations. (j) Structural transformation of SR780 and SR780@Fe in Fe3+ and acidic environments. *** p < 0.001
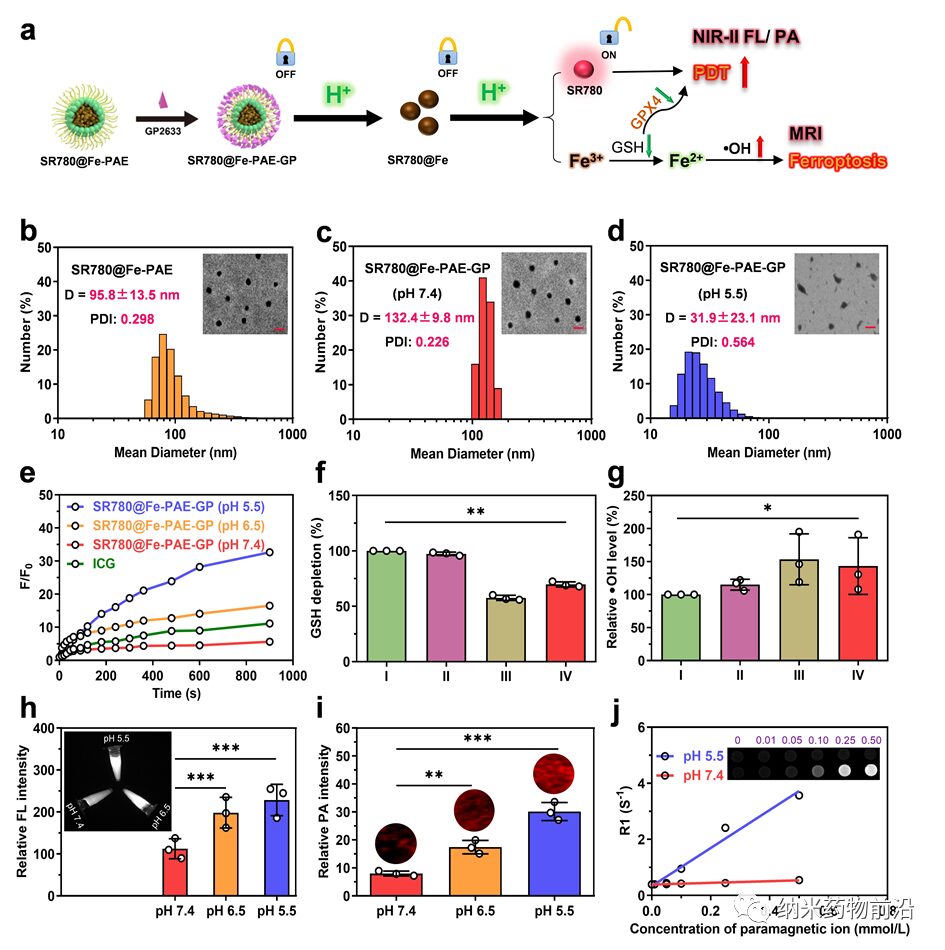
Figure 3. Morphological and property characterization of SR780@Fe-PAE and SR780@Fe-PAE-GP. (a) Schematic of the preparation and pH activation mechanism of SR780@Fe-PAE-GP nanoparticles. (b-d) Particle size distribution of SR780@Fe-PAE, SR780@Fe-PAE-GP, and SR780@Fe-PAE-GP (pH=5.5). Insets: TEM photos of SR780@Fe-PAE, SR780@Fe-PAE-GP, and SR780@Fe-PAE-GP (pH=5.5) (scale bar: 200 nm). (e) ROS generation ability of SR780@Fe-PAE-GP under different pH values (λ = 808 nm, 0.5 W cm−2). (f, g) Investigation of ·OH and GSH levels in different groups (I: PBS, II: SR780, III: SR780@Fe, IV: SR780@Fe-PAE-GP, n = 3) for ferroptosis effects. (h-j) Investigation of NIR-II fluorescence, photoacoustic, and MRI properties of SR780@Fe-PAE-GP under different pH conditions. *p < 0.05, **p < 0.01, ***p < 0.001
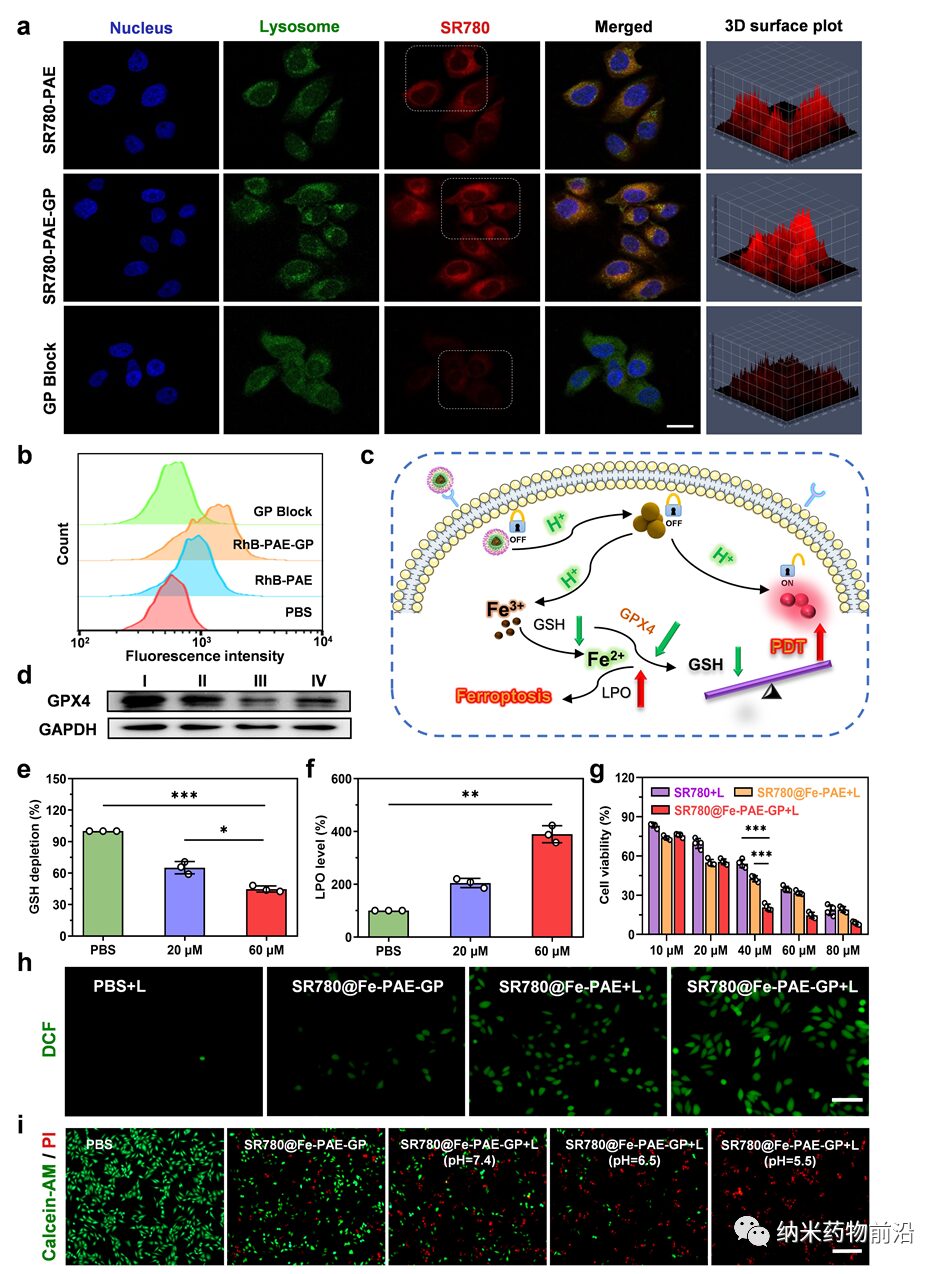
Figure 4. Investigation of nanoparticle endocytosis at the cellular level and the synergistic mechanism of PDT and ferroptosis. (a, b) Endocytosis of nanoparticles by HepG-2 cells at different time points. Scale bar: 20 μm. (c) Schematic of the synergistic mechanism of PDT and ferroptosis. (d) Western blot analysis of GPX4 protein expression in HepG-2 cells treated with different groups (I: PBS, II: SR780-PAE-GP, III: SR780@Fe-PAE, IV: SR780@Fe-PAE-GP). (e, f) Expression levels of GSH and LPO in HepG-2 cells treated with different concentrations of SR780@Fe-PAE-GP. (g) Survival rate of HepG-2 cells after incubation with different concentrations (λ = 808 nm, 0.5 W cm−2, 2 min) of SR780+Laser, SR780@Fe-PAE+Laser, and SR780@Fe-PAE-GP+Laser. (h) Fluorescence images of ROS generation in HepG-2 cells treated with PBS, PBS+Laser, SR780@Fe-PAE-GP, SR780@Fe-PAE+Laser, and SR780@Fe-PAE-GP+Laser (λ = 808 nm, 0.5 W cm−2, 2 min). Scale bar: 50 μm. (i) Live/dead assay images of HepG-2 cells treated with different groups. Scale bar: 100 μm. *p < 0.05, **p < 0.01, ***p < 0.001
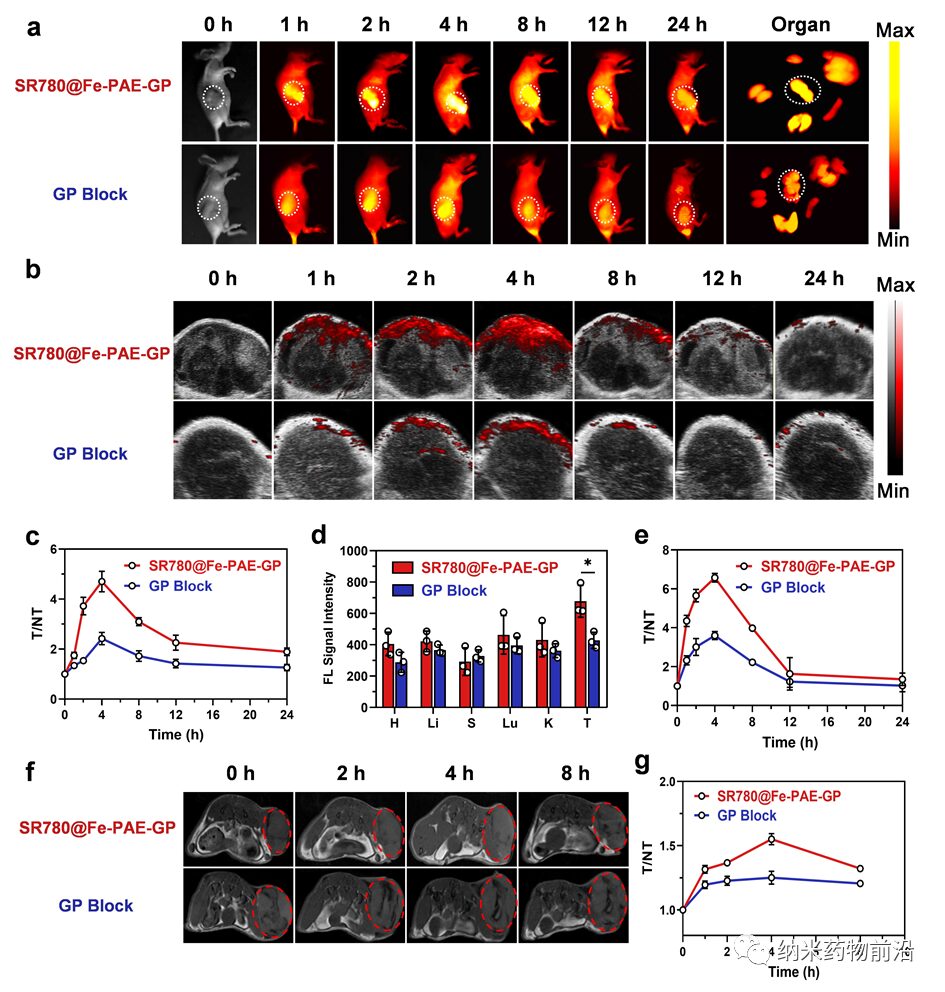
Figure 5. Evaluation of in vivo multimodal imaging effects of SR780@Fe-PAE-GP. (a, b) In vivo NIR-II fluorescence and photoacoustic imaging results at different time points after treatment of PDXHCC model with different groups. (c) Relative NIR-II fluorescence signal intensity at the tumor site at different time points for different groups. (d) NIR-II fluorescence signal intensity of major organs in mice 24 h after administration. (e) Relative photoacoustic signal intensity at the tumor site for different groups at different time points. (f) T1 weighted MRI results at the tumor site for different groups at different time points. (g) Relative intensity of T1 weighted signals at the tumor site at different time points. Data are presented as mean ± standard deviation (n = 3, *p < 0.05).
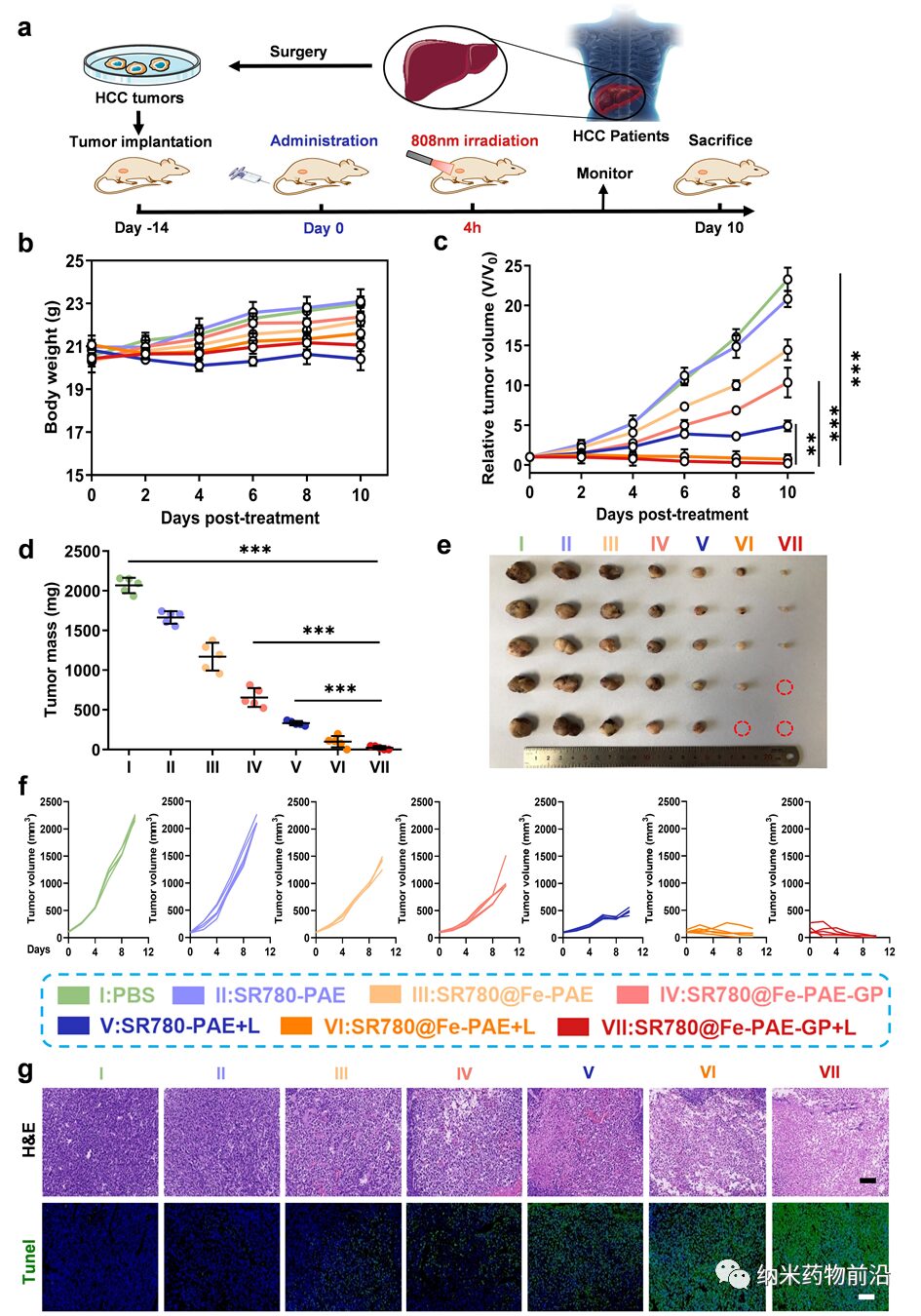
Figure 6. In vivo anti-tumor therapy. (a) Schematic of the establishment of the PDXHCC model and the in vivo treatment process. (b, c) Changes in body weight and relative tumor volume in mice after treatment with different groups. (d, e) Tumor weight and images of mice from different groups after 10 days of treatment. (f) Changes in tumor volume during the treatment process for a single group. (g) H&E and TUNEL staining of tumor tissues from mice treated with different groups. Scale bar: 100 μm. Data are presented as mean ± SD (n = 5; **p < 0.01, ***p < 0.001)
In summary, we designed and synthesized a novel amphiphilic photosensitizer capable of pH-responsive NIR-II fluorescence and photoacoustic imaging, which also exhibits good PDT therapeutic effects. Since it can coordinate with Fe3+ and restore the properties of the photosensitizer under acidic conditions, we selected a pH-responsive polymer and externally connected a peptide that actively targets the surface receptor GPC-3 of liver cancer cells, thereby preparing a pH-responsive active targeting nanoparticle with tumor in situ activation “on/off” functionality. In vitro and in vivo experimental results show that during liver cancer treatment, the nanoparticles not only exhibit multimodal imaging capabilities but also demonstrate a good synergistic effect of PDT and ferroptosis.
This work was supported by the National Natural Science Foundation, Guangdong Natural Science Foundation, and other funding sources. PhD students Sun Rui and Ma Wen from the School of Pharmacy, Southern Medical University, and attending physician Ling Mingjian are co-first authors, and Professor Yu Zhiqiang from Southern Medical University and Researcher Tang Longguang from Zhejiang University are co-corresponding authors.
Corresponding Author Introduction:

Yu Zhiqiang, PhD, Professor, PhD Supervisor, Postdoctoral Co-supervisor. Director of the Laboratory Medicine Center at Dongguan People’s Hospital affiliated with Southern Medical University, and Executive Deputy Director of the Dongguan Clinical Drug Research Institute. He holds a PhD in Pharmacy from Nagasaki University and completed a postdoc at Arizona State University in the USA. He has served as a professor of pharmacy at Southern Medical University and director of the Advanced Biomaterials PI team. His team has long been engaged in integrated cancer diagnosis and treatment, novel targeted nanodrug delivery systems, tumor biomarker screening, and molecular diagnostic reagent development. In recent years, the team has published over 50 SCI papers in international journals such as Adv. Mater, J. Am. Chem. Soc, ACS Nano, Angew, Adv. Funct. Mater, Nano Lett, Adv Sci., Biomaterials, and JCR, with 12 papers being ESI highly cited. Since 2019, the team has published over 20 papers as corresponding authors in journals with IF >10. The total citations of the papers are over 5400, with an H-index of 42. He has led 3 projects funded by the National Natural Science Foundation and several provincial and municipal projects. He serves as a review expert for over 50 mainstream international journals, including Adv Mater, ACS Nano, Adv Funct Mater, Biomaterials, JMC, etc. He is the Vice Chair of the Nanomedicine Branch of the Guangdong Precision Medicine Application Society; Vice President of the Laboratory Economics Branch of the Guangdong Health Economics Association; Vice President of the Molecular Diagnostics Society of the Guangdong Translational Medicine Society; Youth Committee Member of the Nanotechnology Society of China Anti-Cancer Association; Youth Committee Member of the Biological Process Committee of China; Youth Committee Member of the Pharmaceutical Chemistry Professional Committee of China; Editorial Board Member of Chemistry Letters; Youth Editorial Board Member of AJPS; and Youth Editorial Board Member of the Journal of Pharmacy.

Tang Longguang, Researcher at the International Institute of Health and Medicine of Zhejiang University, and the Fourth Affiliated Hospital of Zhejiang University School of Medicine, Master’s Supervisor; selected as an “Innovative Talent” in Zhejiang Province. Deputy Editor of Nano TransMed; Principal Investigator of a National Natural Science Foundation project, a Youth Fund, and a major project in Jinhua City. He obtained his PhD from Xiamen University and studied for two years at the National Institutes of Health (NIH) in the USA under the sponsorship of the China Scholarship Council. His research focuses on the application of biomedical engineering in the diagnosis and treatment of cancer, aging, stroke, and respiratory diseases, dedicated to the clinical translation of novel multifunctional molecular imaging probes, antibody-drug conjugates, nanodelivery carriers, and small molecule drugs. His main research achievements include the development of a series of small molecules and nanodrugs targeting neutrophils for cancer and traumatic brain injury treatment; the design and development of new near-infrared I/II dyes and their self-assembled nanodrugs for tumor PET/fluorescence/photoacoustic imaging and photothermal/photodynamic therapy; and the first report of a small molecule radioactive probe specifically targeting the HER2 receptor for molecular typing and SPECT/CT imaging of breast cancer. To date, he has published over 30 SCI papers in internationally renowned journals in the fields of nanomedicine, nuclear medicine, and medicinal chemistry, of which he has published 19 SCI papers as first/corresponding authors (including co-authors) (15 papers in JCR zone 1), with 12 papers having IF >10 (including one paper with IF >30); the total IF exceeds 320; he has applied for 8 national invention patents, of which 4 have been authorized. He was awarded the Alavi–Mandell Award by the Society of Nuclear Medicine and Molecular Imaging.
First Author Introduction:

Sun Rui, graduated from Bengbu Medical College, and in 2019 was recommended to pursue a PhD in Pharmacy at Southern Medical University. His research interests include integrated research on traditional Chinese medicine nano-formulations and photodynamic nano-formulations for cancer treatment. He has published 2 SCI papers as the first author in J CONTROL RELEASE and J NANOBIOTECHNOL, and co-first authored 1 SCI paper in NANO RES, and has applied for 2 national invention patents.