Recently, Associate Professor Zhang Wenli’s team at China Pharmaceutical University published a review titled “The Janus of Protein Corona on Nanoparticles for Tumor Targeting, Immunotherapy and Diagnosis” in Journal of Controlled Release, Volume 345, 2022, and it was selected as the Inside Back Cover article of that issue..

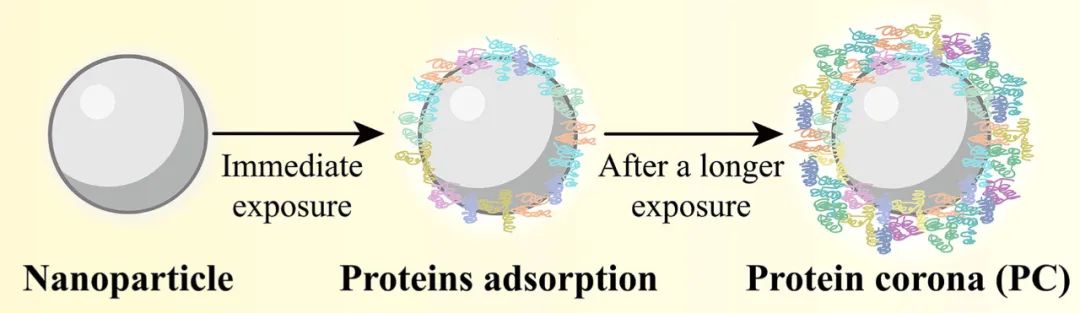
The protein corona refers to a structure composed of one or more layers of proteins that adsorb onto the surface of nanoparticles after they come into contact with biological media. The formation of the protein corona interferes with or regulates the fate of nanoparticles in the body, thus affecting the in vivo and in vitro correlation of data (such as particle size, potential, and drug release rate), making their in vivo behavior difficult to predict.[1-2] Therefore, the study of protein corona is crucial for guiding the rational design of nanomedicines, clarifying their fate in vivo, and promoting their clinical translation.
This article focuses on reviewing the impact mechanisms of protein corona on the targeting process of anti-tumor nanomedicines (in vivo circulation, accumulation and penetration at the tumor site, and uptake by tumor cells) and immunotherapy, exploring how to effectively reduce the formation of protein corona or rationally apply its characteristics during formulation design; it also reviews the research progress of protein corona as a new type of biomarker for tumor diagnosis.
The authors compare the protein corona to the “Janus” god, emphasizing that the protein corona on the surface of nanoparticles can interfere with tumor targeting and immunotherapy, while in some cases, it can also play a promoting role. As a biomarker for tumor diagnosis, it has both advantages and disadvantages. This review aims to provide a more comprehensive in vivo perspective for the design of anti-tumor nanomedicines.
1. Mechanism of Protein Corona Formation on Nanoparticles
Most nanoparticles (including inorganic and organic nanoparticles) can rapidly adsorb proteins to form a protein corona upon contact with biological media (such as plasma and tissue fluid). However, the formation of the protein corona on the surface of nanoparticles is a dynamic and continuous process that spans the entire transport process of nanoparticles in vivo, with two main explanatory theories: the Vroman effect and cooperative adsorption. The former refers to the exchange adsorption of proteins on the surface of nanoparticles, where proteins of higher concentration in biological media first adsorb onto the nanoparticle surface, only to be eventually replaced by proteins with higher affinity; the latter refers to the already adsorbed proteins on the nanoparticle surface providing a scaffold for subsequent protein adsorption [3-4].
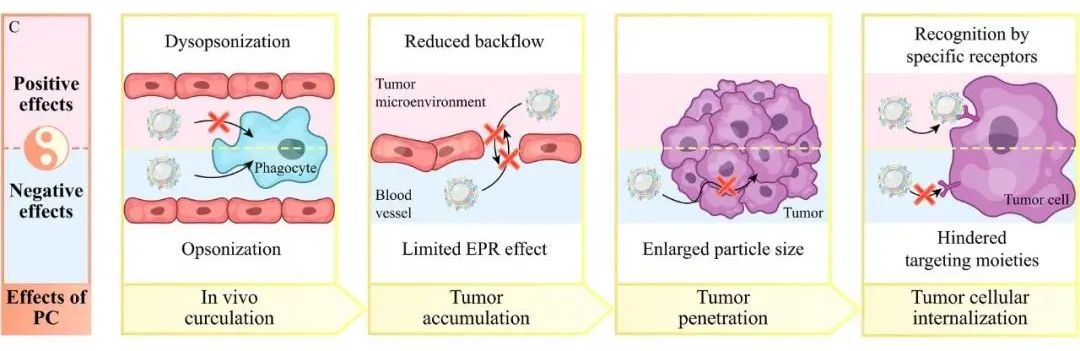
(i) In vivo circulation: If the main components of the formed protein corona are opsonins, such as coagulation proteins, complement, immunoglobulins, and fibrin, it can lead to the recognition and clearance of nanoparticles by the mononuclear phagocyte system; if the main components of the protein corona are dysopsonins, such as apolipoproteins and albumin, it can allow nanoparticles to evade the surveillance of the mononuclear phagocyte system, achieving a longer in vivo circulation time.
(ii) Accumulation at the tumor site: The formation of the protein corona leads to an increase in nanoparticle size, making it difficult for them to extravasate from blood vessels to the tumor site; or after crossing the blood vessels, they may not easily return, which is more favorable for accumulation at the tumor site.
(iii) Penetration of tumor tissue: The formation of the protein corona increases the size of nanoparticles, reducing their penetration capability in tumor tissues (e.g., the binding of poly(lactic-co-glycolic acid) nanoparticles with extracellular matrix proteins alters their size and leads to a decrease in penetration capability [6]); however, the mechanism by which the protein corona enhances nanoparticle penetration still requires further study.
(iv) Uptake by tumor cells: The protein corona can cover modified ligands on the nanoparticle surface or exhibit unfavorable steric effects, thereby reducing the interaction between nanoparticles and cell membranes, leading to decreased uptake; on the other hand, specific proteins in the protein corona can also cause specific pathways of cellular internalization by recognizing receptors on tumor cell membranes (e.g., apolipoprotein E in the protein corona can recognize low-density lipoprotein receptors highly expressed on Hep G2 tumor cells, resulting in increased nanoparticle uptake [7]).
Based on this, to improve the tumor targeting efficiency of nanoparticles, one can address the dual impact of protein corona. On one hand, by using PEG modification, zwitterionic coatings (such as sulfonated trimethyl ammonium ethyl ester and poly(methacrylic acid) phosphatidylcholine), and biological membrane coatings to encapsulate nanoparticles, the formation of protein corona can be weakened; on the other hand, nanoparticles can be modified to adsorb specific proteins in vivo (e.g., modifications of polyphosphate favor the adsorption of apolipoprotein A-I and apolipoprotein J [8]) or pre-encapsulated with specific proteins in vitro (such as albumin, transferrin, and lactoferrin) to form an artificial protein corona, enhancing their tumor targeting efficiency.
-
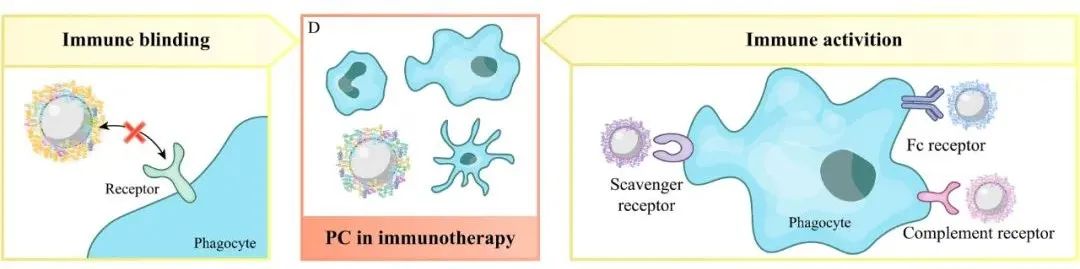
3. The Impact and Application of Protein Corona Formation on Tumor Immunotherapy
-
4. Protein Corona as a New Type of Biomarker for Tumor Diagnosis
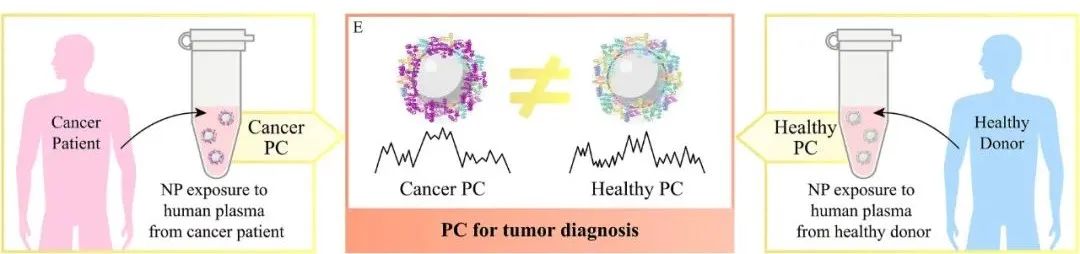
Different types of tumors have been shown to express different specific proteins, leading to changes in the concentrations of corresponding proteins in plasma, which results in differences between plasma derived from tumor patients and healthy volunteers [10]. When nanoparticles are incubated in plasma samples from tumor patients, specific proteins in the plasma will be adsorbed and concentrated on the surface of nanoparticles, forming a cancer protein corona. Therefore, by detecting the protein corona formed on the surface of nanoparticles, changes in the composition and concentration of proteins in plasma caused by tumor growth and invasion can be identified, enabling early detection of tumors.
However, the composition of the protein corona can also be influenced by other diseases in patients, which may interfere with the accuracy of tumor diagnosis results; meanwhile, there is still a significant lack of protein corona databases for different tumor types based on the same nanoparticle diagnostic platform, thus the application value of protein corona as a biomarker for tumor diagnosis still needs further exploration.
5. Summary and Outlook
After nanoparticles enter the biological body, the protein corona formed on their surface will affect their fate in vivo and produce a dual effect like the “Janus” in tumor targeting, tumor immunotherapy, and tumor diagnosis. It is important to note that the composition of the protein corona has species differences, therefore, it is encouraged to use appropriate human-derived cells or blood samples for protein corona research, which will help accurately understand the impact or application of protein corona on nanoparticles in the human body, thereby narrowing the gap between preclinical research and clinical trials. At the same time, the protein corona will also affect the clinical translation of nanoparticles in tumor detection and treatment, thus it is necessary to re-evaluate the impact of protein corona formation on the efficacy and safety of already approved clinical nanomedicines..
-
1. Reviewed the dual-edged role of protein corona in anti-tumor targeting therapy and immunotherapy;
-
2. Summarized the research progress and advantages and disadvantages of protein corona as a new type of biomarker for tumor diagnosis;
-
3. Discussed how to effectively reduce the formation of protein corona or rationally apply its characteristics during formulation design.

Wang Xiaobo

Zhang Wenli
Extracellular Fate Regulation of Exosomes: A New Frontier for Nanomedicine
Hydroxycholesterol Enhances LNPs-mRNA Transfection Efficiency in T Cells
Lactoferrin-Modified PLGA Nanoparticles Enhance the Blood-Brain Barrier Permeability and Neuroprotective Effects of Resveratrol
Bibliometric and Visualization Analysis of Ocular Drug Delivery Research
Inflammatory Features of COVID-19 and Their Impact on Drug Delivery
Challenges in Peptide and Protein Drug Delivery: Solutions Based on Silk Fibroin
Selectively Controlled Release Polymer Prodrug Nano-Assembly System for Targeted Tumor Therapy
Cover Story: Future Blueprint of Next-Generation Cancer Vaccines
Recent Advances in Multifunctional Reverse-Lyotropic Liquid Crystals
Circular RNA: An Emerging Therapeutic Target for Drug and Vaccine Therapy
More Exciting Content, Please Scan to Follow
